Abstract
Radical forequarter amputation is often performed for recurrent proximal extremity tumors. A free forearm fillet flap is used to provide excellent coverage of the resultant defect without donor site morbidity. Use of a free flap from the distal portion of the extremity with proximal tumor burden is safe and effective.
Introduction
The creation of a donor site from a free flap is not without morbidity, especially in a patient already undergoing major extirpative surgery. The free fillet flap may be a practical option when reconstructing this type of defect in an extremity. This flap is often characterized using the ‘spare parts’ notion, which employs the principle of utilizing viable and undamaged tissue from amputated limbs that would otherwise be discarded. These flaps are most commonly utilized after severe traumatic injuries to the extremities where replantation is contraindicated, however, their description in the setting of malignant neoplasms is lacking [Citation1–8].
Although fillet flaps are more commonly implemented in reconstruction of the lower extremity [Citation4,Citation6,Citation7], their successful use in the hand, forearm and shoulder have been reported [Citation1,Citation2,Citation4,Citation5,Citation9]. This is likely because of narrower replantation indications for the lower extremity [Citation3,Citation4] or the high frequency of limb sparing tumor resections in the upper extremity [Citation10]. Nevertheless, there are several benefits to fillet flaps including elimination of donor site morbidity and immediate coverage of the wound [Citation2,Citation6,Citation11]. The authors describe the use of this flap in salvage forequarter amputations for recurrent osteosarcoma of the upper extremity.
Case I
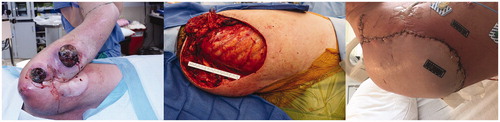
A 57-year-old man presented for salvage resection of a recurrent osteosarcoma of the right shoulder and chest wall (). He had previously undergone neoadjuvant radiation therapy followed by resection and coverage with a free anterolateral thigh flap anastomosed to the thoracodorsal vessels. On physical exam, the patient had two large fungating masses overlying the right proximal shoulder. Magnetic resonance imaging revealed the masses to be in close proximity to the axillary vessels with metastases to the lungs. In order to decrease tumor burden and increase the patient’s quality of life, a forequarter amputation was planned. Intra-operatively, the orthopedic oncology team completed the tumor resection while the plastic surgery team dissected out the brachial artery with elbow disarticulation, isolating the vascular pedicle. After amputation and vessel ligation, the wound measured 1200 cm2 with exposed subclavian vessels. On the back table, a cuff incision was made on the forearm at the distal wrist and extended vertically onto the dorsum. The flap was filleted open with subperiosteal elevation along the radius and ulna, which were then resected. Microvascular anastomosis proceeded in the standard fashion utilizing an end-to-side technique from the brachial artery into the subclavian artery and brachial vein into the subclavian vein. Perfusion was confirmed via indocyanine green fluorescence. The patient healed with no complications at with no cancer recurrence in the flap, but eventually succumbed to his disease.
Case II
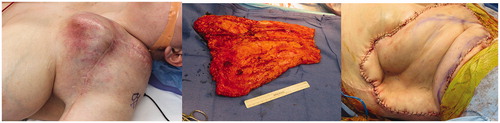
A 30-year-old female presented with a second recurrence of high-grade sarcoma of the left supraclavicular region (). She had previously undergone radical resection of a proximal humeral osteosarcoma with implantation of a reverse total shoulder arthroplasty, complicated by recurrence two years later. This was radically resected with removal of the endoprosthesis and reconstructed with a pedicled latissimus dorsi muscle flap. A multi-disciplinary surgical team planned for re-resection and reconstruction with a free forearm fillet flap. Prior to tumor resection, the flap was elevated as a fasciocutaneous flap from distal to proximal utilizing a stocking-seam incision with inclusion of ulnar and radial arteries. As there was an expected delay with several hours of cold ischemia time, muscle was not included in the forearm fillet flap. Avoiding the inclusion of muscle reduced blood loss and allowed for an increased ischemia time with decreased reperfusion injury. The internal mammary artery and vein were chosen as recipient vessels as the tumor abutted the proximal axillary vessels. Radical tumor resection was performed resulting in forequarter amputation with a defect measuring 1000 cm2. After negative margins were confirmed, microvascular anastomosis was performed in an end-to-end manner, connecting the brachial artery and cephalic vein to the internal mammary artery and vein, respectively. The flap was trimmed and inset, and perfusion confirmed with indocyanine green fluorescent imaging. The patient healed with no complications at with no cancer recurrence in the flap, but eventually succumbed to her disease.
Discussion
The concept of spare parts reconstruction is frequently utilized in traumatic extremity injuries, as it provides stable, vascularized coverage to a large wound while eliminating concomitant donor site morbidity [Citation7,Citation11–15]. However, the literature is limited in describing the safety of this technique in oncologic cases, specifically in cases of recurrent tumors. In these cases, local/regional and free tissue transfer procedures may have already been utilized, leaving a lack of donor sites. Patients with recurrent tumor also tend to have poor long-term survival rates, making the lack of functional donor-site morbidity an ideal option when considering fillet flaps.
In the upper extremity, the forearm can be easily circumferentially dissected, or ‘filleted’, to create a composite bulky flap [Citation6,Citation7,Citation12]. This is especially advantageous in large defects with significant dead space. The vessels tend to be of large caliber with dependable perfusion to the flap; these sizeable vessels make for a technically easier anastomosis resulting in reduced operating time [Citation2,Citation5,Citation14]. While the large flap size is advantageous, venous drainage can sometimes be limited. This can be resolved by ‘supercharging’ the venous outflow, in which case particular attention should be paid to vein selection. The venous drainage of the forearm is divided into superficial and deep systems. The cephalic and basilic veins are the major superficial veins of the forearm and the vena comitantes make up the deep supply. Ichinose et al. describes the efficacy of a dual venous anastomosis when separate venous systems are used, reporting a significant reduction in venous thrombosis and ultimately the risk of flap failure [Citation16].
There are few absolute contraindications to the use of a free forearm fillet flap, including tumor extension into the distal forearm and prolonged ischemia time [Citation5,Citation12,Citation17,Citation18]. Patients undergoing a fillet flap for a severe traumatic injury are more susceptible to prolonged donor limb ischemia due to the inherent time spent during tumor resection. Ver Halen et al. describe attempts to limit ischemia by temporarily ‘banking’ the flap by vascular anastomosis to an alternative site, such as the groin, or re-vascularizing the tumor in the middle of the procedure before tumor resection was complete [Citation19]. The senior authors modified the traditional fillet flap in the second case by taking it as a fasciocutaneous flap to allow for an increased ischemia time with decreased reperfusion injury. Relative contraindications include donor site infection, lymphedema and peripheral vascular disease [Citation14,Citation19]. These patients are at increased risk for infection, poor wound healing and edema [Citation20].
Baccarani et al. described a treatment algorithm for the management of the amputated upper extremity, in which fillet flaps remain the first choice of reconstruction [Citation21]. Alternatives to the fillet flap for forequarter amputations include rotational pedicled myocutaneous flaps from the distal humerus [Citation22] and traditional free flaps from other areas of the body. Local options could include chimeric flaps based off the sub-scapular system to increase the surface area that can be covered [Citation23]. However, for patients with oncologic history, local rotational flaps often are not available due to tumor burden, and traditional free flaps are avoided due to significant donor site morbidity. Fillet flaps not only have the benefit of no donor site morbidity but also can be performed with two teams with an acceptable complication profile [Citation24].
In our series, both patients were reconstructed after resection of recurrent tumors. While these patients often have poor prognosis, both cases prove that use of the fillet flap is oncologically sound, as there was no cancer recurrence in either flap.
Conclusion
The free forearm fillet flap is a viable option for reconstruction after forequarter amputation for recurrent upper extremity sarcomas. As few studies have reported its use in the management of large complex defects following high-grade tumor resection, this case series serves to expand the literature on an infrequent reconstructive technique.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Lin CH, Webb K, Neumeister MW. Immediate tissue transplantation in upper limb trauma: spare parts reconstruction. Clin Plast Surg. 2014;41(3):397–406.
- Oliveira IC, Barbosa RF, Ferreira PC. The use of forearm free fillet flap in traumatic upper extremity amputations. Microsurgery. 2009;29(1):8–15.
- Baek R-M, Eun S-C, Heo C-Y, et al. Amputation stump salvage using a free forearm flap from the amputated part. J Plast Reconstr Aesth Surg. 2009;62(10):e398–e400.
- Cavadas PC, Raimondi P. Free fillet flap of the hand for elbow preservation in nonreplantable forearm amputation. J Reconstr Microsurg. 2004;20(5):363–366.
- Hammond DC, Matloub HS, Kadz BB, et al. The free-fillet flap for reconstruction of the upper extremity. Plast Reconstr Surg. 1994;94(3):507–512.
- Flurry M, Melissinos EG, Livingston CK. Composite forearm free fillet flaps to preserve stump length following traumatic amputations of the upper extremity. Ann Plast Surg. 2008;60(4):391.
- Küntscher MV, Erdmann D, Homann H-H, et al. The concept of fillet flaps: classification, indications, and analysis of their clinical value. Plast Reconstr Surg. 2001;108(4):885.
- Mohammed F, Romany S, Ramdass MJ, et al. A pedicle forearm fillet flap aided by subperiosteal proximal dissection in shoulder disarticulation. Plast Reconstr Surg. 2002;109(7):2431.
- Scaglioni MF, Lindenblatt N, Barth AA, et al. Free fillet flap application to cover forequarter or traumatic amputation of an upper extremity: a case report. Microsurgery. 2016;36(8):700–704.
- Bickels J, Kollender Y, Malawer M. Forequarter amputation. operative techniques in orthopaedic surgical oncology. 2nd ed. Philadelphia (PA): Wolters Kluwer; 2012.
- Lee GK, Mohan SV. Complex reconstruction of a massive shoulder and chest wall defect: de-bone appétit flap. J Surg Case Rep. 2010;2010(3):1–1.
- Sakamura R, Nohira K, Shibata M, et al. Coverage of a large soft-tissue defect of the chest with a free fillet forearm and hand flap. J Reconstr Microsurg. 2001;17(4):229–232.
- Tukiainen E. Chest wall reconstruction after oncological resections. Scand J Surg. 2013;102(1):9–13.
- Tran NV, Evans GRD, Kroll SS, et al. Free filet extremity flap: indications and options for reconstruction. Plast Reconstr Surg. 2000;105(1):99.
- Richards A, Klaasen M, Parkhouse N. The free-fillet flap for reconstruction of the upper extremity. Plast Reconstr Surg. 1995;96(2):488–491.
- Ichinose A, Terashi H, Nakahara M, et al. Do multiple venous anastomoses reduce risk of thrombosis in free-flap transfer? Efficacy of dual anastomoses of separate venous systems. Ann Plast Surg. 2004;52(1):61.
- Zachary LS, Gottlieb LJ, Simon M, et al. Forequarter amputation wound coverage with an ipsilateral, lymphedematous, circumferential forearm fasciocutaneous free flap in patients undergoing palliative shoulder-girdle tumor resection. J Reconstr Microsurg. 1993;9(02):103–107.
- Schmidt RG, Springfield DS, Dell PC. Chest wall reconstruction with a free extended forearm flap. A case report. J Reconstr Microsurg. 1987;3(3):189–191.
- Ver Halen JP, Yu P, Skoracki RJ, et al. Reconstruction of massive oncologic defects using free fillet flaps. Plast Reconstr Surg. 2010;125(3):913.
- Pace M, Gattai R, Matteini M, et al. Toxicity and morbility after isolated lower limb perfusion in 242 chemo-hyperthermal treatments for cutaneous melanoma: the experience of the Tuscan Reference Centre. J Exp Clin Cancer Res. 2008;27(1):67.
- Baccarani A, Follmar KE, De Santis G, et al. Free vascularized tissue transfer to preserve upper extremity amputation levels. Plast Reconstr Surg. 2007;120(4):971–981.
- Shah SA, Wong WH, Adhvaryu D. Rotational pedicle myocutaneous forearm fillet flap used to fill forearm amputation defect: indications and uses. J Hand Surg. 2018;43(4):390.e1.
- Wu WC, Chang YP, So YC, et al. The combined use of flaps based on the subscapular vascular system for limb reconstruction. Br J Plast Surg. 1997;50(2):73–80.
- Kim JY, Subramanian V, Yousef A, et al. Upper extremity limb salvage with microvascular reconstruction in patients with advanced sarcoma. Plast Reconstr Surg. 2004;114(2):400–408.