Abstract
Here we describe the case of a 60‐year‐old-woman with systemic sclerosis sent to our Scleroderma Unit to treat digital stumps. The stumps were successfully treated with autologous fat grafting (crown-shape infiltration). Our technique of autologous lipotransfer improved wound healing in a scleroderma patient with stump-digital ulcers where all other options failed.
Introduction
Digital ulcers (DUs) represent one of the most severe and frequent manifestations of systemic sclerosis (SSc) occurring in almost 50% of SSc patients [Citation1–7]. Moreover, DUs are painful, recurrent, hard to heal, they can become infected and evolve into gangrene, risking amputation [Citation8]. This surgical technique results in a negative impact on digital function and deeply affect patients’ quality of life. Taking into account that skin ulcers and amputations represent a clinical challenge, a combined treatment (medical, surgical and physiotherapy) is mandatory. The adipose tissue is recognised as an easy to access source, and stem cell-based therapies have emerged as a promising option to improve DUs healing too because of their broad range of clinical applications [Citation9–14]. Autologous fat grafting (AFG) provides trophic effects and could have beneficial effects for the treatment of SSc skin ulcers. Up to now, there is no data on the treatment of amputation in scleroderma patients.
We are reporting a case of a SSc patient treated with AFG injection for the treatment of long term stump skin lesions arriving at the 24-week follow up period.
Case presentation
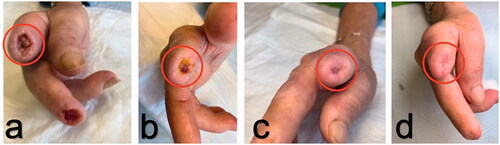
A 60‐year‐old woman affected by SSc with recurrent digital ulcers was sent to our Scleroderma Unit for the treatment of the amputation stumps resistant to traditional therapies. The disease was characterised by Raynaud phenomenon, telengiectasias on hands and face, oesophageal and interstitial lung involvement. She was positive to serum antinucleolar antibodies and presented a capillaroscopic scleroderma pattern. During 2018, the patient developed multiple DUs unresponsive to systemic therapies (calcium-channel blockers, prostanoids and/or anti-ET receptors). The ulcer on the II finger of the right hand became particularly severe, showing irregular margins with exposure of both the flexor tendon and phalangeal bone, and even the digital ulcer on the III finger worsened. Amputation of both distal and proximal phalanges of the II finger together with the amputation of the distal phalanges of the III finger was performed in a surgical department. Despite topical therapy and antibiotics (i.e. co-amoxiclav, flucloxacillin and ciprofloxacin) used to treat the recurrent infections, the DUs on the stumps continued to increase in size and depth with exudate, and became more painful (VAS: 9/10) even though analgesics (paracetamol and opioids) were used. 3 months following surgical intervention, a further amputation was proposed, and as a consequence the patient visited our outpatient department for DUs management. A global approach including both systemic (incremental use of intravenous prostanoids) and local treatment (advance wound care) was initiated as previously described [Citation15]. A slight reduction in the size of the DUs on II and III fingers was observed over this time, but they continued to cause pain and produce discharge, with bone exposition.
In May 2019, treatment with AFG was performed as additional therapy for the treatment of the stump DUs. Adipose tissue was obtained from the peri-umbilical region after infiltration with a modified Klein solution (50 ml of saline solution, 0.5 ml of 1:1.000 adrenalin and 10 ml of 2% Mepivacain). In detail, fat harvesting was performed using an aspiration cannula (Micro Aspiration Cannulas Black & Black Surgical FAC, 12 gauge × 15 cm Luer-lock), introduced through a 3 mm incision. The required amount of lipoaspirate was estimated using a standard formula: 2 cc lipoaspirate per square centimeter wound size and approximately 15 ml of fat and fluids was aspirated in 10 ml Luer-lock syringes. No centrifugation of the lipoaspirate was performed. The lipoaspirate was then transferred into 1-cc Luer-lock syringes using a closed system (Luer Lock Adapter), followed by infiltration of the lipoaspirate using a 2 × 80-mm Supra Luer Lock cannula into the wound edges and subdermal plane all around the finger stump (crown-shape infiltration). From 0.5 to 1 ml of fat was grafted at least in each finger stump. The finger stump was then covered with a sterile wound dressing. The patient received perioperative and post-operative antibiotic therapy. No side-effects related to the fat grafting injection were recorded and no infections were observed. The only reaction reported was mild pain at the lipoaspiration site, which was rapidly resolved using escin topical cream, and global pain in the ulcer site too (VAS: 6/10) in the days following AFG, requiring transient increase in analgesic therapy (paracetamol, fentanyl). Finger stiffness was also reported after the fat microinjection. The patient displayed neither clinical inflammation nor pain during any of the subsequent weekly visits, merely a mild oedema at the site of the lipoaspirate injection.
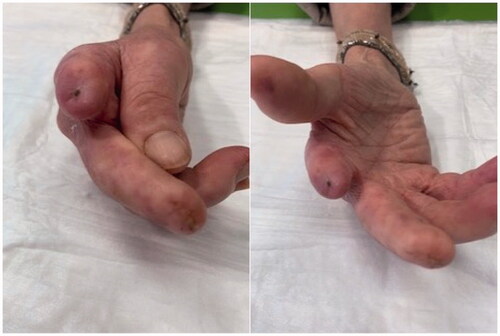
After the first month of AFG treatment, the DUs gradually improved showing re-epithelialization from the margins and granulation of the wound bed; by the end of the 3-month treatment period they had all completely healed. Daily use of analgesics was gradually reduced and discontinued within 2 months, as expression of AFG efficacy in pain management too. These remarkable results remained stable during the 3 and 6 month clinical observations. Three months after AFG, no other amputations were needed (). The patient is still under regular clinical observation in order to determine the long term effects of AFG ().
Discussion
The results of our report suggested that AFG treatment represents an important approach to the scleroderma stump skin ulcers responsible for severe chronic pain and marked reduction of patients’ quality of life. In addition, the treatment with fat grafting was significantly more effective than systemic vasoactive therapy and traditional topical approach and it was well tolerated. The treatment of scleroderma DUs is challenging, particularly for lesions complicated by local infections and gangrene that compromise the outcome of skin lesions in a significant percentage of patients, often requiring surgical amputation [Citation16]. Stump skin ulcers are difficult to treat and many of treatment are anecdotally supported. As far as we know, this is the first report showing the healing of the stump-DUs in a scleroderma patient using AFG.
Autologous fat grafting is frequently used for soft tissue defect reconstruction and it becomes a relatively common procedure due its availability, low rate of adverse events and relative ease of harvesting. Several modifications have been made to the procedures of fat harvesting, processing, and injecting [Citation17]. Fat tissue is usually obtained from the flanks, peri-umbilical region, or internal side of the thigh or knee. Low negative-pressure aspiration with large-bore cannulas minimize adipocyte damage during fat harvesting. According to other authors in our case was used the ‘wet’ method of fat harvesting which involves fluid injection at the donor site and facilitates lipoaspiration, while minimizing pain and ecchymosis. For fat processing, centrifugation at a low speed is preferable to high-speed centrifugation, gravity separation or filtration [Citation17,Citation18]. No centrifugation of the lipoaspirate was performed in our cases as suggested by other authors to prevent fat damage [Citation19]. After decantation, fat injection was performed using small-gauge cannulas in a fanning out pattern over multiple sessions, rather than a single session. Fat is gently distributed above the dorsal deep fascia to avoid perforation of the vessels. Treatment of stump skin ulcers in scleroderma patients is poorly investigated even though it is a severe and invalidating symptom, mainly in patients with digital gangrene.
The usefulness of AFG in the treatment of stump skin lesions was reported firstly by Bourne et al. [Citation20], and confirmed by Malik et al. [Citation21], in patients with traumatic lower extremity amputations.
The reason why local fat grafting may induce such positive changes in SSc stump skin ulcers remains almost completely speculative at the moment.
Some studies have shown that stem cells in fat tissue are multipotent cells potentially able to differentiate themselves into other cell types, like cells osteogenic, myogenic, endothelial under the conditioning of different stimulant, as demonstrated in a lot of in vitro and in vivo studies. There is also an evidence that progenitor cells may play a role in fat grafting. It is well established that multiple cell types, including mature adipose cells, endothelial progenitor cells, pericytes, and adipose-derived stromal cells are present in harvested adipose tissue [Citation22–24]. Several studies have demonstrated the regenerative powers of AFG, probably related to the production of growth factors such as vascular endothelial growth factor (VEGF), keratinocyte growth factor (KGF) and other factors which lead to keratinocyte migration and acceleration of healing. AFG may also directly stimulate the production from other resident cell types of a number of cytokines with immune-modulating action probably capable of downregulating some pathological mechanisms in injured tissues [Citation25–29]. Since SSc is a systemic endothelial disorder characterized by a reduction of capillary flow and the consequent fibrotic evolution, it could be postulated that AFG may be involved in releasing a number of growth factors and cytokines which stimulate the angiogenesis and the formation of new capillary loops, able to the heal stump DUs as observed in our study.
The present study confirmed previous data suggesting that local transfer of autologous may be a successful option to induce healing in ischemic SSc related fingertip DUs that are resistant to more traditional therapeutic approaches [Citation30,Citation31]. Clinical improvements observed in our patient on DUs after AFG are definitely in line with more recent reports on the treatment of fibrosis of the skin and wound healing [Citation32–37].
In conclusion, our report suggests that the use of AFG is effective and safe for the treatment of SSc manifestations as stump skin ulcers and related pain. Nevertheless, further clinical and experimental studies are needed, in order to better understand the exact mechanisms of action of stem cells and to standardize this approach.
Disclosure statement
The authors report no conflicts of interest.
References
- Elhai M, Meune C, Avouac J, et al. Trends in mortality in patients with systemic sclerosis over 40 years: a systematic review and meta-analysis of cohort studies. Rheumatology. 2012;51:1017–1026.
- Ioannidis JPA, Vlachoyiannopoulos PG, Haidich AB, et al. Mortality in systemic sclerosis: an international meta-analysis of individual patient data. Am J Med. 2005;118:2–10.
- Ferri C, Valentini G, Cozzi F, et al., Systemic Sclerosis Study Group of the Italian Society of Rheumatology (SIR-GSSSc). Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore). 2002;81:139–153.
- Van den Hoogen F, Khanna D, Fransen J, et al. 2013 Classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65:2737–2747.
- Hachulla E, Launay D. Diagnosis and classification of systemic sclerosis. Clin Rev Allergy Immunol. 2011;40:78–83.
- Ferri C, Sebastiani M, Lo Monaco A, et al. Systemic sclerosis evolution of disease pathomorphosis and survival. Our experience on Italian patients’ population and review of the literature. Autoimmun Rev. 2014; 13:1026–1034.
- Walker UA, Tyndall A, Czirják L, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials and Research Group database. Ann Rheum Dis. 2007;66:754–763.
- Steen V, Denton CP, Pope JE, et al. Digital ulcers: overt vascular disease in systemic sclerosis. Rheumatology (Oxford). 2009;48:iii19–iii24.
- Coleman SR. Structural fat grafting: more than a permanent filler. Plast Reconstr Surg. 2006;118:108S–120S.
- Rigotti G, Marchi A, Galie M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007; 119:1409–1422. discussion 1423-4.
- Klinger M, Caviggioli F, Klinger FM, et al. Autologous fat graft in scar treatment. J Craniofac Surg. 2013;24:1610–1615.
- Juhl AA, Karlsson P, Damsgaard TE. Fat grafting for alleviating persistent pain after breast cancer treatment: a randomized controlled trial. J Plast Reconstr Aesthet Surg. 2016; 69:1192–1202.
- Coleman SR, Katzel EB. Fat grafting for facial filling and regeneration. Clin Plast Surg. 2015; 42:289–300, vii.
- Rigotti G, Charles-de-Sá L, Gontijo-de-Amorim NF, et al. Expanded stem cells, stromal-vascular fraction, and platelet-rich plasma enriched fat: comparing results of different facial rejuvenation approaches in a clinical trial. Aesthet Surg J. 2016;36:261–270.
- Kowal-Bielecka O, Landewé R, Avouac J, et al., EUSTAR Co-Authors. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR). Ann Rheum Dis. 2009;68:620–628.
- Giuggioli D, Manfredi A, Colaci M, et al. Scleroderma digital ulcers complicated by infection with fecal pathogens. Arthritis Care Res (Hoboken). 2012;64:295–297.
- Coleman SR. Hand rejuvenation with structural fat grafting. Plast Reconstr Surg. 2002; 110:1731–1744. discussion 1745-7.
- Pu LL, Coleman SR, Cui X, et al. Autologous fat grafts harvested and refined by the Coleman technique: a comparative study. Plast Reconstr Surg. 2008;122:932–937.
- Fantozzi F. Hand rejuvenation with fat grafting: a 12-year single-surgeon experience. Eur J Plast Surg. 2017;40:457–464.
- Bourne DA, Thomas RD, Bliley J, et al. Amputation-site soft-tissue restoration using adipose stem cell therapy. Plast Reconstr Surg. 2018;142:1349–1352.
- Malik P, Gaba S, Ahuja C, et al. Role of fat graft alone versus enriched fat graft with stromal vascular filtrate in painful amputation stump. Indian J Orthop. 2019;53:452–458.
- Gimble JM, Katz AJ, Bunnel BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260.
- Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther. 2017;8:145
- Simonacci F, Bertozzi N, Raposio E. Off-label use of adipose-derived stem cells. Ann Med Surg (Lond). 2017;24:44–51.
- Frazier T, Alarcon A, Wu X, et al. Clinical translational potential in skin wound regeneration for adipose-derived, blood-derived, and cellulose materials: cells, exosomes, and hydrogels. Biomolecules. 2020;10:1373.
- Denton CP. Systemic sclerosis: from pathogenesis to target therapy. Clin Exp Rheumatol. 2015; 33:3–7.
- Capelli C, Zaccara E, Cipriani P, et al. Phenotypical and functional characteristics of in vitro-expanded adipose-derived mesenchymal stromal cells from patients with systematic sclerosis. Cell Transplant. 2017; 26:841–854.
- Magalon J, Velier M, Simoncini S, et al. Molecular profile and proangiogenic activity of the adipose-derived stromal vascular fraction used as an autologous innovative medicinal product in patients with systemic sclerosis. Ann Rheum Dis. 2019;78:391–398.
- Manetti M. Could autologous adipose-derived stromal vascular fraction turn out an unwanted source of profibrotic myofibroblasts in systemic sclerosis? Ann Rheum Dis. 2020;79:e55.
- Del Papa N, Di Luca G, Andracco R, et al. Regional grafting of autologous adipose tissue is effective in inducing prompt healing of indolent digital ulcers in patients with systemic sclerosis: results of a monocentric randomized controlled study. Arthritis Res Ther. 2019;21:7.
- Del Papa N, Di Luca G, Sambataro D, et al. Regional implantation of autologous adipose tissue-derived cells induces a prompt healing of long lasting indolent digital ulcers in patients with systemic sclerosis. Cell Transplant. 2015; 24:2297–2305.
- Scuderi N, Ceccarelli S, Onesti MG, et al. Human adipose-derived stromal cells for cell-based therapies in the treatment of systemic sclerosis. Cell Transplant. 2013; 22:779–795.
- Granel B, Daumas A, Jouve E, et al. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: an open-label phase I trial. Ann Rheum Dis. 2015;74:2175–2182.
- Strong AL, Rubin JP, Kozlow JH, et al. Fat grafting for the treatment of scleroderma. Plast Reconstr Surg. 2019;144:1498–1507.
- Chen B, Wang X, Long X, et al. Supportive use of adipose-derived stem cells in cell-assisted lipotransfer for localized scleroderma. Plast Reconstr Surg. 2018; 141:1395–1407.
- Pignatti M, Spinella A, Cocchiara E, et al. Autologous fat grafting for the oral and digital complications of systemic sclerosis: results of a prospective study. Aesthetic Plast Surg. 2020;44:1820–1832.
- Bene MD, Pozzi MR, Rovati L, et al. Autologous fat grafting for scleroderma-induced digital ulcers. An effective technique in patients with systemic sclerosis. Handchir Mikrochir Plast Chir. 2014;46:242–247.