Abstract
We studied 21 patients who underwent radical ameloblastoma excision followed by immediate reconstruction. Comorbidities, consumption of alcohol and/or tobacco and BMI status did not contribute to an unfavorable outcome. Giant ameloblastoma (≥5 cm) and/or tumor involving bony curvatures increased surgical complexity, the incidence of complications and hospital stay.
Introduction
Ameloblastoma is a rare head and neck tumor with an estimated annual incidence of 0.5 per million population [Citation1,Citation2]. They constitute 1% of tumors and cysts involving the jaws and accounts for approximately 10% of the odontogenic tumors [Citation3]. Ameloblastomas are originated from the epithelial lining of odontogenic cysts, enamel organ or dental lamina, stratified epithelium of oral cavity or displaced epithelial remnants [Citation4,Citation5]. They are primarily seen in adults during the third and fourth decade of life with no gender preference and more frequently located in the mandible (80%), especially in the angle and ascending ramus [Citation6].
Even though they are benign and slow-growing lesions, ameloblastomas exhibit locally destructive behavior with a high recurrence rate [Citation5]. Thus, most relapses (50% and even over 80%) occur during the first 5 years after the primary surgery [Citation3,Citation7]. The major contributing factor for recurrence seems to be the inadequate initial surgical procedure rather than the histological type [Citation8,Citation9].
The surgical options for ameloblastoma vary from simple enucleation (with or without bony curettage) to radical excision. The histological infiltration of these tumors beyond macroscopic and even radiological boundaries force the creation of safety margins to prevent recurrences [Citation10]. Thus, tumor enucleation and curettage may cause not only an unacceptable likelihood of recurrence but also an increasing risk of fractures due to the maintenance of unhealthy and/or weakened bony structures [Citation11]. Considering undetectable microscopic spreading, especially through the central cancellous bone, the radical surgical excision appears as the only modality of treatment with a reasonable curative rate for both primary and recurrent ameloblastomas [Citation4,Citation12]. Because of that, immediate defect reconstruction, mandibular or maxillary bony stabilization, dental rehabilitation and even sensory restoration also require the attention of the surgical team.
Since we have an unusually high consultation for ameloblastoma, this study reviews our comprehensive multidisciplinary management and the potential variables that influenced the long-term outcomes during a period of 5 years.
Materials and methods
This is a retrospective analysis based on medical records of patients with histopathological confirmation of ameloblastoma that were managed by our multidisciplinary team between June 2015 and June 2020. We obtained institutional review board approval (protocol #2020-0039) for this single-institution study.
We collected relevant demographics (i.e. age, gender, race, etc.), comorbidities and their therapeutic interventions, alcohol consumption, tobacco/marijuana use and body mass index (BMI). Characteristics of ameloblastoma clinical presentation (location, size, symptoms, histology, previous surgeries, etc.) were also reviewed. In addition, we compiled information on preoperative assessment (i.e. imaging studies, virtual surgical planning, blood tests, etc.), types of ablative and reconstructive surgeries, antibiotics and deep venous thrombosis (DVT) prophylaxes and nutritional assistance as well as operative complications, length of stay, secondary rehabilitation procedures and long-term outcomes.
All our patients had preoperative panoramic dental radiograph (OPT), CT scan of head and neck and incisional biopsy. Of note, definitive surgeries were typically done within a month after the initial evaluation. For the histopathological examination, we used the WHO classification that describes four different variants: solid/multicystic, unicystic, desmoplastic and extraosseous/peripheral [Citation13]. The two more prominent tumor dimensions in the preoperative CT scans were used to establish their sizes. In addition, to design the surgical reconstruction, tumor locations were categorized into central (lesion between canines), lateral (tumor affecting the mandibular body, angle and/or ramus), central-lateral (lesion located in central and lateral regions), hemimandible (extensive tumor on one half of the mandible) and bilateral (extensive tumor affecting both sides of the mandible) [Citation14]. We also requested computed tomography angiogram (CTA) in cases where vascularized bone grafts such as fibula free flap (FFF) and scapula tip-free flap (STFF) were considered to reconstruct the secondary defects. Virtual surgical planning (VSP) sessions were carried out to quantify the magnitude of the resection (including additional safety margins) and design the features of the bony structure and internal fixation required for the reconstructive procedure. When possible, we also revised the surgical strategies for inferior alveolar nerve (IAN) grafting and intraoperative placement of dental implants.
According to the clinical evaluation, patients were managed with radical resection with safety margins of 1.5-cm of uninvolved bone around the lesion. To repair the secondary defects, plating alone was reserved for elderly patients (edentulous with atrophic mandible) and/or the presence of medical conditions that contraindicated more complex management. For the remaining patients, customized reconstruction plates were preoperatively bent to be used along with either non-vascularized (from iliac crest) or vascularized bone grafts (i.e. FFF and STFF). In general, vascularized bone grafts were used in bony defects larger than 6-cm (including tumor and surrounding safety margins). Tracheostomy, local flaps, submandibular gland removal and IAN grafting were done according to the patients’ needs. The immediate or delayed placement of dental implants was pursued upon approval by the patients’ financial assistance.
Data are presented as means ± standard deviation (SD). When appropriate, the median values are also displayed. The nonparametric Mann–Whitney U test was used for statistical analysis of two samples. The Kruskal–Wallis test was used for statistical analysis of more than two independent samples. A p-value of ≤0.05 was considered statistically significant. The analysis was performed with a commercially available software package GraphPad InStat 3 (GraphPad Software, San Diego, CA).
Results
This study included 21 patients, 13 males and 8 females (ratio of 1.6:1). Their age ranged between 14 and 68 years (mean 38.9 ± 20 years). The study group consisted of 18 African American (85.7%) and 3 Caucasian (14.3%) patients. Their lesions had an average duration of 28.9 months prior to the surgery (median: 12 months). With the exception of one case, ameloblastomas were located in the mandible (95.2%). In this regard, the mandibular location was primarily lateral (66.7%) with a slight predominance of the right side (57.1%). Central was the second more common site (14.3%) followed by central-lateral and bilateral with 9.5% each. The main reason for consultation in our clinic was painless swelling or mass (66.7%) followed by paresthesias and local pain (19% each). Other clinical manifestations included facial asymmetry, malocclusion and mobile teeth. In 3 cases, the diagnosis was incidental during routine dental appointments (). The tumor size during the preoperative assessment was on average 5.3 ± 2.2 cm by 3.8 ± 1.7 cm. Five patients had a history of previous surgical attempts namely curettage (9.5%), enucleation (9.5%) and drainage (4.8%). The histopathological evaluation revealed multicystic (57.1%), unicystic (38.1%) and desmoplastic (4.8%) variants. No peripheral variant was found in our study group.
Table 1. Ameloblastoma: clinical presentation.
Interestingly, 16 out of 21 patients (76.2%) presented at least one significant comorbidity under permanent treatment at the time of the surgery. In fact, 42.9% of our patients presented two or more comorbidities (). High blood pressure and diabetes mellitus were the more frequent pre-existing medical conditions followed by coronary artery disease, cancer and gout (). Concomitantly, high values of body mass index (BMI) were seen in a considerable number of our patients. Thus, 13 patients exhibited BMI above 30 (61.9%) and six of them above 35 (28.6%). The mean BMI was 30.4 ± 7.4 with a median of 31.6 (range between 19.2 and 48.8). In addition, regular consumption of alcohol, tobacco and marijuana were confirmed in 28.6%, 23.8% and 9.5% of our cases, respectively.
Table 2. Comorbidities in ameloblastoma patients.
As part of the preoperative protocol, evaluation by anesthesia and other specialties (as needed) were requested. We routinely ordered blood tests (i.e. hemoglobin, white blood cells, glucose, creatinine, PTT, INR, others) which were usually within or close to normal range, and therefore, they did not affect our decision-making process and surgical schedule.
Only 17 patients (81%) required tracheostomy since surgical procedures of four cases did not represent a risk for airway obstruction. Mandibular ameloblastomas were removed with either segmental resection or hemimandibulectomy. The maxillary ameloblastoma was managed with partial maxillectomy (). As part of the ablative procedure, the resection of submandibular gland and teeth extraction were often included. Plating alone was performed in 2 patients (9.5%). These patients were edentulous and/or with underlying medical conditions that contraindicated any complex reconstruction (). The secondary defects of the remaining patients were repaired using reconstruction plate associated with either non-vascularized (14.3%) or vascularized bone grafts (76.2%). The non-vascularized bone grafts were obtained from the iliac crest and used in cases where the full specimens (tumor and safety margins) were smaller than 6 cm and the resulting defects were surrounded by good quality soft tissue and healthy bone (). Complex bony reconstruction with vascularized bone grafts were used in larger tumors. Thus, we performed fibula free flap in 15 cases and scapula tip-free flap in one case (). Within FFF, donor sites were chosen from left legs in 8 patients (53.3%) and right legs in the remaining 7 patients (46.7%). These patients required one (33.3%), two (13.3%) or three fibular segments (53.3%) to recreate the resected bony contour (). The decision to perform one or more fibular segments in the FFF was based on tumor size (p-value of 0.02) and/or the need to remove either the angle or anterior part of the mandible (p-value of 0.002) because of tumor location or to reach safety margins. Variables such as the presence of comorbidities, chronic use of medications or BMI status did not affect the complexity of the fibular construct.
Figure 1. (A) A 66-year-old edentulous male with a 3 × 2.6 cm2 multicystic ameloblastoma of the left mandibular body. Patient consulted 4 months after onset of progressive painless mass. Several comorbidities (high blood pressure, gout, prostate cancer) and permanent use of 6 medications. Chronic consumption of tobacco (1 ppd for 44 years) and alcohol (1–2 beers weekly). BMI of 33 kg/m2. (B) Ameloblastoma was treated with left segmental mandible resection, reconstruction plate and left IAN grafting. Length of stay of 1 day. No postoperative complications.
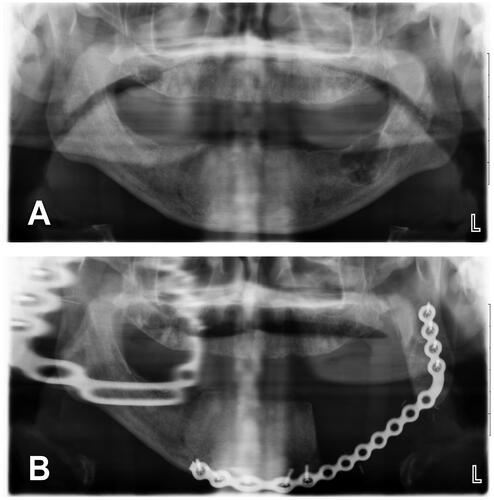
Figure 2. (A, B) A 17-year-old male with 3.4 × 2 cm2 multicystic ameloblastoma of the left posterior mandibular body. Patient consulted 20 months after onset of progressive painless mass. History of high blood pressure treated with two antihypertensive agents. No history of tobacco or alcohol consumption. BMI of 41 kg/m2. (C, D) Ameloblastoma was treated with left segmental mandible resection, non-vascularized bone graft from iliac crest, reconstruction plate and left IAN grafting. No tracheostomy was required. Length of stay of 4 days. No postoperative complications.
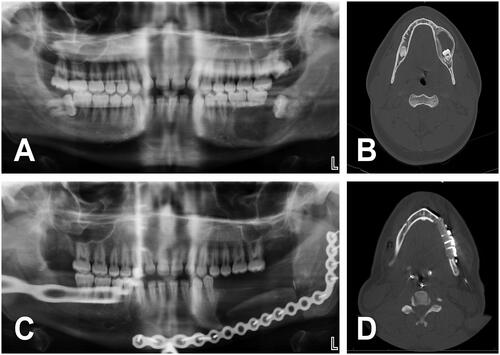
Figure 3. (A) A 14-year-old male with 5.3 × 4 cm2 multicystic ameloblastoma of the right posterior body and ramus of the mandible. Patient asymptomatic until tumor was detected in a routine dental appointment. History of chronic sinusitis treated with anti-allergic agents. No tobacco or alcohol consumption. BMI of 22 kg/m2. (B) Virtual surgical planning was performed prior to the surgery. (C, D) Ameloblastoma was treated with tracheostomy, right hemimandibulectomy, right submandibular gland resection, right fibula free flap (two segments) with reconstruction plate and right IAN grafting (green arrowhead). (e) Prior to discharge, CT scan facial bones with 3D reconstruction was performed to check the FFF construct. Length of stay of 12 days. (F–H) No postoperative complications except hypertrophic scars of submandibular and tracheostomy incisions.
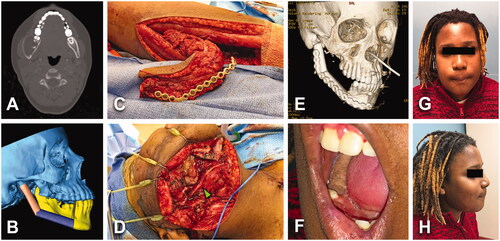
Table 3. Summary of surgical procedures.
In 11 patients, split-thickness skin grafts were needed to complete coverage of donor sites, especially after FFF procedures. The scapula tip-free flap was selected for an ameloblastoma case sitting in the posterior part of the right maxilla. The complexity of the bony reconstruction and the need for a long pedicle made this flap an excellent choice. The donor site was closed primarily and the patient did not experience any long-term functional problem ().
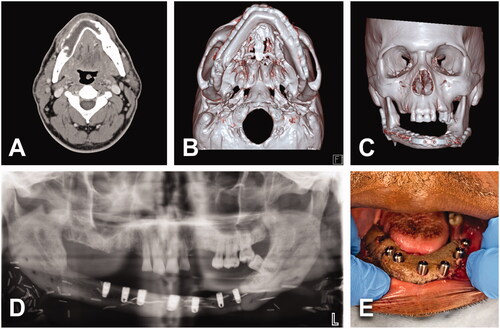
Figure 4. (A) A 54-year-old male with an asymptomatic 2.8 × 2.4 cm2 unicystic ameloblastoma of the right posterior maxilla (green arrowhead). History of high blood pressure treated with 2 antihypertensive agents. No tobacco/alcohol consumption. BMI of 22 kg/m2. (B) Virtual surgical planning helped to design a two-segments construct from scapular tip free flap. (C) Patient underwent to right partial maxillectomy via intraoral approach and right STFF. No tracheostomy was required. (D) Postoperative 3D CT scan showed stable bony structure. Length of stay of 6 days. (E–G) No major postoperative complications, but intraoral flap debulking was required to normalize occlusion.
A submental artery island flap was necessary in 2 cases of plating only to restore the intraoral lining (9.5%). The inferior alveolar nerves (IAN) were repaired with allograft nerves in 15 cases (71.4%), four of them performed bilaterally. Even though our experience is limited, the sensory recovery and patients’ satisfaction have been promising. A detailed assessment of our AIN grafting results will be submitted in a separate manuscript.
Multiple dental implants were done in 6 of our patients (28.6%), half of them during the primary procedures and the remaining half in a delayed fashion ( and ). The indication for dental implant placement and their timing was mainly related to the financial situation and/or health insurance assistance of our patients. Candidates went through a specific assessment for this purpose including virtual surgical planning.
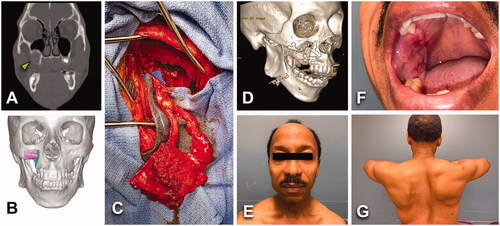
Figure 5. (A, B) A 17-year-old female with an asymptomatic 3.5 × 1.9 cm2 unicystic ameloblastoma of anterior mandible (green arrowhead). No past-medical history or tobacco/alcohol consumption. BMI of 25 kg/m2. (C–E) Lesion was treated with segmental mandible resection, reconstruction plate, non-vascularized bone graft from left iliac crest. No tracheostomy was required. Length of stay of 2 days. No postoperative complications. (F) Four months after primary surgery, patient got 2 tapered screw-vent (3.7 × 11.5) dental implants in sites 23 and 26.
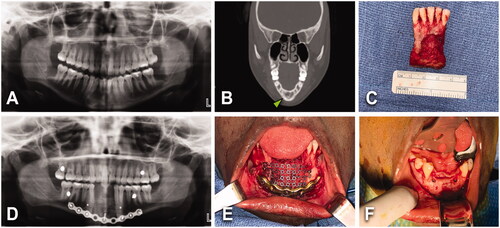
Figure 6. (A) A 51-year-old male with 7.9 × 2.9 cm2 multicystic ameloblastoma of the right mandibular body, extending across midline up to left body. No history of comorbidities; however, tobacco consumption (0.5 ppd for 30 years). BMI of 33 kg/m2. (B, C) Lesion was treated with tracheostomy, segmental mandible resection, right submandibular gland resection, 3 segments right FFF and bilateral IAN grafting. Length of stay of 11 days. Patient had delayed healing of donor site at the right leg. (D, E) One year after primary surgery, patient got multiple dental implants. Patient experienced intraoral dehiscence and exposure of reconstruction plate responding satisfactorily to hardware removal.
Ten of our patients (47.6%) exhibited giant ameloblastoma (≥5 cm in length) during the initial assessment with a mean size of 7.2 ± 1.5 × 5.2 ± 1.5 cm [Citation9,Citation15]. The remaining patients (<5 cm in length) had a mean tumor size of 3.5 ± 0.8 × 2.47 ± 0.6 cm. Giant ameloblastomas not only increased the complexity of the reconstruction by forcing the indication of FFF, but they also presented a higher incidence of postoperative complications (60%) compared to that in patients with tumors <5 cm in size (36.4%). This difference was statistically significant with a p-value of 0.04. In this regard, within the giant tumor category (n = 10), one patient had a single complication while five patients experienced two complications. Furthermore, 77% of our surgical complications were developed in ‘giant tumor’ patients. Compared to patients with smaller tumors, this frequency was also statistically significant (p-value of 0.03). Interestingly, the postoperative complications were not associated with the presence and/or the number of comorbidities (p-value of 0.65), continuing use of medications (p-value of 0.29) and the number of these medications (p-value of 0.46). In addition, we could not find any correlation between tobacco use or alcohol consumption and the presence of postoperative complications (each variable with a p-value of 0.22). The small number of marijuana users in our study group prevented us to reach any conclusion about its role in postoperative complications.
No thrombotic events (DVT and flap thrombosis) were seen in our patients. In this regard, enoxaparin (90.5%) and aspirin (28.6%) were indicated as prophylactic therapy along with sequential compression devices (SCDs) on non-surgical legs.
Antibiotic prophylaxis was achieved with intravenous administration of sulbactam-ampicillin (76.2%), cefazolin (14.3%) or clindamycin (9.5%). In general, these antimicrobial agents were maintained until the removal of neck drains. Despite these precautions, five patients (23.8%) developed infectious complications, three of them at surgical sites (). In addition, delayed healing of donor sites, especially those for FFF harvest, was common to see (28.6%). This complication did not interfere with our discharge plans; however, it increased the number of visits to the outpatient clinic and the need for further debridement procedures and secondary skin grafts. Interestingly, these patients did not develop major long-term problems to walk. Poor preoperative nutrition and/or postoperative deterioration were not seen in our study group, and therefore, they were no contributing factors for infection or delayed healing. In fact, when necessary, nutritional assistance was implemented via nasogastric Dobhoff tube (81%) or percutaneous endoscopic gastrostomy (4.8%).
Table 4. Postoperative surgical complications.
Another relevant complication was the bulkiness of intraoral skin paddle especially important in patients pursuing dental rehabilitation. These patients required debulking procedures to optimize the local condition (). Other complications exhibited a low incidence rate. For instance, the orocutaneous fistula responded well to surgical debridement and oral antibiotics. One patient that received multiple dental implants in a delayed fashion (one year after primary surgery) developed intraoral dehiscence and exposure of the reconstruction plate. This complication was successfully managed with surgical debridement and hardware removal ().
In our study group, the average length of stay (LOS) was 9.5 ± 5.4 days (median: 9 days). In this regard, the LOS was 7.9 ± 5.8 days in tumors with <5 cm and 11.2 ± 4.6 days in tumors with ≥5 cm in size. This difference was statistically significant with a p-value of 0.02. The ameloblastoma management with FFF compared to other reconstructive procedures significantly delayed the discharge from the hospital (p-value of 0.001); however, no differences on LOS were seen between FFF with one versus three bony segments. Interestingly, the LOS was not affected by the presence and number of comorbidities (p-value of 0.2). In addition, the BMI did not impact the LOS regardless of the type of analysis. Thus, when comparing different BMI categories (<25, 25–30, 30–35 and 35+), between BMI <30 and BMI 30+ subgroups or between BMI <35 and BMI 35+ subgroups, no statistical differences were found (p-value of 0.8, 0.4 and 0.2, respectively).
At the time of this report, the postoperative period of our patients was on average of 25.2 ± 16.9 months (median of 20 months). None of our patients have presented local recurrence or developed distant metastases.
Discussion
Ameloblastoma is a benign lesion of the jaws with a locally invasive course and high recurrence rate when is not satisfactorily resected [Citation3,Citation5,Citation7]. They are seen, more frequently, in dark-skinned people and in developing countries [Citation7]. Interestingly, metastasizing ameloblastomas occur in 2% of the cases where metastatic deposits develop especially in lungs and cervical lymph nodes [Citation16–19]. In contrast to metastasizing ameloblastomas, which maintain a benign profile as in primary tumors [Citation16–19], ameloblastic carcinomas show histological features of aggressive malignant epithelial odontogenic tumors [Citation20,Citation21].
In general, options for surgical treatment of ameloblastoma include a conservative approach (i.e. enucleation, curettage) for small lesions and a radical approach, namely wide bone resection followed by secondary defect reconstruction, in cases of large tumors. Interestingly, ameloblastomas exhibit a high incidence of recurrence not because of their dimensions or histological types, but their insufficient local excision [Citation8,Citation9]. Thus, even though enucleation has been reported as an appropriate treatment for unicystic lesions, we performed wide bone resection in all cases. This radical surgical approach is supported by the fact that a definitive histological type is regularly confirmed only with a comprehensive postoperative examination of specimens. For instance, the intramural variants of the ‘traditionally benign’ unicystic lesions have a significantly higher recurrence rate than the luminal variants and they are difficult to differentiate from each other without permanent histological sections [Citation22–24]. The use of specific molecular markers for tumor identification, cell proliferation rate and migration capacity can help establish not only the accurate diagnosis but also the prognosis in terms of tumor aggressiveness and potential for recurrence [Citation25]. Interestingly, unicystic lesions have the same mutational profile as conventional ameloblastomas opening the possibility that they represent different clinical and histological degrees of the same disease [Citation26]. Ameloblastoma cells can also express markers of dental epithelial stem cells (i.e. SOX2 and BMI1), and therefore, they have the potential to transdifferentiate into cancer stem cells and subsequently be responsible for tumor relapse [Citation27]. The fragmentation of primary tumors during conservative surgical procedures resulting in the seeding of ameloblastoma cells on wound beds may also contribute to increasing the recurrence risk [Citation13]. In this regard, the findings of a recent meta-analysis study also favor radical management for both multicystic and unicystic ameloblastomas [Citation28]. Therefore, radical local resection with a 1.5-cm margin of healthy bone around the lesion and immediate reconstruction seems to be the more comprehensive treatment for ameloblastomas in terms of disease control as well as appropriate functional and cosmetic outcomes [Citation4,Citation12].
Our study group seems to be still small to make definitive statements. However, unlike other published reports, we collected our cases in a single institution during a significantly shorter period of time (five years). This situation created high consistency of existing resources, team composition and surgical protocol to manage these lesions.
As previously reported, our ameloblastoma cases affected mainly the mandible of males and within the African American community. Due to multiple causative factors that are beyond the scope of this study (i.e. socioeconomic, level education, rurality condition, etc.), our patients consulted in our clinic several months after the onset of symptoms and with locally advanced lesions. Our preoperative assessment was often simple and rapid (i.e. basic bloodwork, OPT, CT scan head and neck, anesthesia assessment) so that patients could be taken to the operating room within few weeks. As mentioned above, we managed all our ameloblastoma cases with radical local resection followed by immediate reconstruction. The ablative portion also included secondary procedures as needed (). Reconstruction with a plate alone was chosen in edentulous patients and/or with pre-existing medical conditions that contraindicated complex procedures (). Virtual surgical planning sessions were crucial to reduce the operative time and enhance the accuracy of our reconstruction in cases where either non-vascularized or vascularized bone grafts were chosen. In fact, these sessions allowed us to estimate the number of bony segments, measure their sizes and pre-bend the reconstruction plates. As seen in and , non-vascularized bone grafts from the iliac crest were used when the total resection defects (tumor and safety margins) were smaller than 6 cm in length and located within one mandibular region [Citation14]. The resulting defects were surrounded by well-vascularized soft tissues and healthy bone to secure graft uptake. In concordance with other publications, our cases of non-vascularized bone grafts provided adequate structure and contour for dental implants associated with the short length of stay and low incidence of secondary procedures [Citation24]. In addition, they presented no complications such as infection, delayed healing, excessive bony resorption, fractures or plate exposure.
One maxillary and 15 mandibular ameloblastomas were reconstructed with STFF and FFF, respectively. The STFF was an ideal reconstructive option with regard to constructing shape and pedicle length to treat an ameloblastoma lesion located in the posterior part of the right maxilla. This patient achieved excellent functional and cosmetic outcomes without evident donor site complications (). As expected, the complexity of the FFF construct was linked to large lesions (especially giant ameloblastomas) and the need to re-establish the natural contour of the mandible. Thus, the reconstruction of the angle and anterior portion of the mandible required two and three fibular segments, respectively ( and ). The surgical treatment with FFF was a factor that inevitably prolonged the length of stay regardless of their complexity in terms of the number of fibular segments. Interestingly, we were unable to establish any correlation between increased stay-in-hospital and several common variables of our population such as the presence of co-morbidities, chronic use of medications, high levels of BMI and consumption of alcohol and/or tobacco. We speculate that, due to their high prevalence in our clinical practice, these variables are no longer contributing factors in extending the LOS.
In order to improve the functional and cosmetic outcomes as well as patients’ satisfaction, we incorporated dental rehabilitation with osseointegrated implants and inferior alveolar nerve repair with nerve allografts to our comprehensive treatment. These positive experiences will be reported in an upcoming manuscript.
The immediate postoperative period was focused on maintaining the patient’s airway, flap monitoring, early detection of bleeding or hematoma, prevention of infection and thrombotic events, restarting feeding when possible and improving the patient’s physical condition for a quick and safe discharge from the hospital.
As mentioned in the results section, the subgroup of giant tumors accumulated not only the more complex reconstructive procedures and length of stay but also the higher number of postoperative complications. Therefore, our efforts will be to focus on increasing awareness of this disease in our population for earlier diagnosis and management as well as periodical follow-up of treated cases for detection of local recurrence or metastasizing lesions. Based on compiling evidence from multiple reports, we are planning to pursue tumor surveillance for at least 5 years [Citation29–31].
Conclusions
The management of ameloblastomas requires a multidisciplinary team that covers not only the ablative part but also the reconstructive, oral rehabilitation (dental implants, mastication, deglutition, speech, etc.), social and emotional aspects of this disease. A long-term commitment and collaborative effort from the team, as well as patients and their families, are crucial to optimize outcomes.
Ethical approval
This study was approved by the Institutional review board (protocol #2020-0039).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- McClary AC, West RB, McClary AC, et al. Ameloblastoma: a clinical review and trends in management. Eur Arch Otorhinolaryngol. 2016;273(7):1649–1661.
- Adeel M, Rajput MSA, Arain AA, et al. Ameloblastoma: management and outcome. Cureus. 2018;10(10):e3437.
- Olaitan AA, Arole G, Adekeye EO. Recurrent ameloblastoma of the jaws. Int J Oral Maxillofac Surg. 1998;27(6):456–460.
- Rapidis AD, Andressakis DD, Stavrianos SD, et al. Ameloblastomas of the jaws: clinico-pathological review of 11 patients. Eur J Surg Oncol. 2004;30(9):998–1002.
- Ghandhi D, Ayoub AF, Pogrel MA, et al. Ameloblastoma: a surgeon’s dilemma. J Oral Maxillofac Surg. 2006;64(7):1010–1014.
- Fregnani ER, da Cruz Perez DE, de Almeida OP, et al. Clinicopathological study and treatment outcomes of 121 cases of ameloblastomas. Int J Oral Maxillofac Surg. 2010;39(2):145–149.
- Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol. 1995;31B(2):86–99.
- Infante-Cossio P, Prats-Golczer V, Gonzalez-Perez LM, et al. Treatment of recurrent mandibular ameloblastoma. Exp Ther Med. 2013;6(2):579–583.
- Gravvanis A, Koumoullis HD, Anterriotis D, et al. Recurrent giant mandibular ameloblastoma in young adults. Head Neck. 2016;38(S1):E1947–E1954.
- Gardner DG, Pelcak AMJ. The treatment of ameloblastoma based on pathologic and anatomic principles. Cancer. 1980;46(11):2514–2519.
- Bianchi B, Ferri A, Ferrari S, et al. Mandibular resection and reconstruction in the management of extensive ameloblastoma. J Oral Maxillofac Surg. 2013;71(3):528–537.
- Hong J, Yun PY, Chung IH, et al. Long-term follow up on recurrence of 305 ameloblastoma cases. Int J Oral Maxillofac Surg. 2007;36(4):283–288.
- Hertog D, Bloemena E, Aartman IHA, et al. Histopathology of ameloblastoma of the jaws; some critical observations based on a 40 years single institution experience. Med Oral Patol Oral Cir Bucal. 2012;17(1):e76–e82.
- Jewer DD, Boyd JB, Manktelow RT, et al. Orofacial and mandibular reconstruction with the iliac crest free flap: a review of 60 cases and a new method of classification. Plast Reconstr Surg. 1989;84(3):391–403.
- Moro A, Pelo S, Gasparini G, et al. Virtual surgical planning for reconstruction of giant ameloblastoma of the mandible. Ann Plast Surg. 2020;85(1):43–49.
- Verneuil A, Sapp P, Huang C, et al. Malignant ameloblastoma: classification, diagnostic, and therapeutic challenges. Am J Otolaryngol. 2002;23(1):44–48.
- Amzerin M, Fadoukhair Z, Belbaraka R, et al. Metastatic ameloblastoma responding to combination chemotherapy: case report and review of the literature. J Med Case Rep. 2011;5:491.
- Luo DY, Feng CJ, Guo JB. Pulmonary metastases from an Ameloblastoma: case report and review of the literature. J Craniomaxillofac Surg. 2012;40(8):e470–e474.
- Hosalkar R, Saluja TS, Swain N, et al. Prognostic evaluation of metastasizing ameloblastoma: a systematic review of reported cases in literature. J Stomatol Oral Maxillofac Surg. 2020. DOI:10.1016/j.jormas.2020.07.001.
- Gunaratne DA, Coleman HG, Lim L, et al. Ameloblastic carcinoma. Am J Case Rep. 2015;16:415–419.
- Salami A, Ezenkwa U, Salami M, et al. Malignant ameloblastoma: a challenging diagnosis. Autops Case Rep. 2018;8(4):e2018043.
- Philipsen HP, Reichart PA. Unicystic ameloblastoma. A review of 193 cases from the literature. Oral Oncol. 1998;34(5):317–325.
- Pogrel MA, Montes DM. Is there a role for enucleation in the management of ameloblastoma? Int J Oral Maxillofac Surg. 2009;38(8):807–812.
- Chae MP, Smoll NR, Hunter-Smith DJ, et al. Establishing the natural history and growth rate of ameloblastoma with implications for management: systematic review and meta-analysis. PLoS One. 2015;10(2):e0117241.
- Jeyaraj P. The dilemma of extensive unilocular radiolucent lesions of the jaws - value of immunohistochemistry as a diagnostic marker and prognostic Indicator. Ann Diagn Pathol. 2019;40:105–135.
- Heikinheimo K, Huhtala JM, Thiel A, et al. The mutational profile of unicystic ameloblastoma. J Dent Res. 2019;98(1):54–60.
- Pagella P, Caton J, Meisel CT, et al. Ameloblastomas exhibit stem cell potential, possess neurotrophic properties, and establish connections with trigeminal neurons. Cells. 2020;9(3):644.
- Hendra FN, Natsir Kalla DS, Van Cann EM, et al. Radical vs conservative treatment of intraosseous ameloblastoma: systematic review and meta-analysis. Oral Dis. 2019;25(7):1683–1696.
- Papaioannou M, Manika K, Tsaoussis B, et al. Ameloblastoma of the mandible with pulmonary metastases 45 years after initial diagnosis. Respirology. 2009;14(8):1208–1211.
- Eckardt AM, Kokemüller H, Flemming P, et al. Recurrent ameloblastoma following osseous reconstruction-a review of twenty years. J Craniomaxillofac Surg. 2009;37(1):36–41.
- Aramanadka C, Kamath AT, Kudva A. Recurrent ameloblastoma: a surgical challenge. Case Rep Dent. 2018;2018:8271205.
