Abstract
We report the case of a forest ranger who developed a polymicrobial infection with Mucor circinelloides after traumatic forearm amputation. Based on our case report we discuss epidemiology and management of this rare and potentially fatal infection.
Introduction
Most commonly fungal osteomyelitis is associated with aspergillus or candida species [Citation1,Citation2].
Osteomyelitis with mucor species is a rare and exceptionally life-threatening condition as mucor spores cause angioinvasive infections in patients with immunosuppressive conditions such as poorly controlled diabetes mellitus, malignancies, neutropenia, transplants, and chronic renal failure [Citation3]. Mucor osteomyelitis is most commonly associated with trauma or surgical intervention [Citation1,Citation4]. A hematogenous spread causing osteomyelitis is extremely rare [Citation4]. There is a male-to-female ratio of > 2:1 in Mucormycosis of bones and joints [Citation2].
Case presentation
A 38-year-old Patient was transported to our emergency department by air ambulance after an accidental traumatic forearm amputation. He was at work as a forest ranger when a steel rope hit his arm. The amputated distal forearm was correctly wrapped in saline-saturated gauze in a plastic bag cooled on ice and brought to our emergency room (). The initially applied tourniquet by paramedics was immediately released, and the decision for replantation was made together with the patient. Broad spectrum antibiotic treatment was established. During an 8-h surgery, we managed to replant the arm with sufficient arterial inflow and venous outflow. Intraoperatively, multiple samples for microbiological testing were taken. Two days later the patient was taken back to the theatre where an additional arterial and venous anastomosis were performed with two saphenous vein grafts. The large skin defect was covered with Coldex skin substitute. Unfortunately, on day 7 after replantation the patient developed progressive swelling, increasing skin necrosis, and venous congestion. An emergent re-exploration took place. Intraoperatively multiple thrombosis of all blood vessels was demonstrated with avital extensor and flexor muscles with visible additional superficial fungal contamination, especially in the distal aspects of the wound ().
Figure 1. A shows the amputated right forearm of the 38-year-old patient from a palmar view in the operating theatre before replantation. B demonstrates the corresponding x-ray.
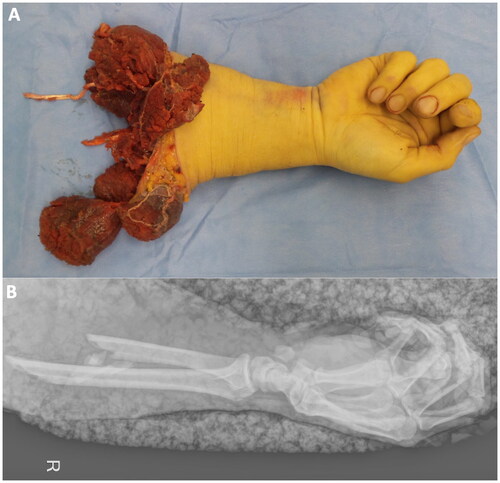
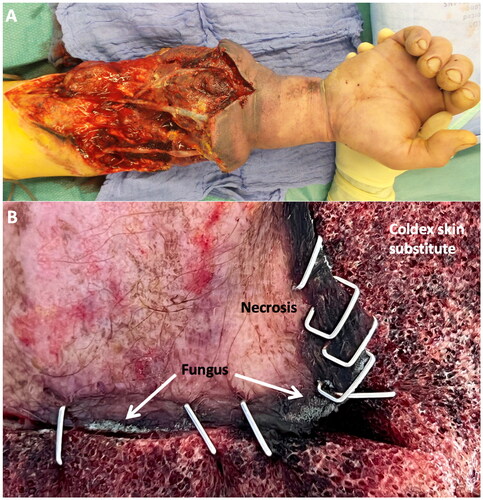
Figure 2. A shows swelling and venous congestion accompanied by necrotic wound areas of the distal forearm 7 days after replantation owing to partially thrombosed arterial and vein grafts. B is a close-up view of necrotic wound edges showing fungus adjacent to the Coldex skin substitute.
In order to save the patient’s life, eventually the decision had to be taken to amputate at the level of the proximal forearm. At that time additional systemic antifungal therapy (Amphotericin B) was implemented, according to our guidelines.
Repeated surgical debridement was performed for the following 14 days. Multiple microbiologic and histologic probes were gained for further investigation. They revealed polymicrobial infection with Bacillus cereus complex, Yersinia enterocolitica group, Staphylococcus capitis, Pseudomonas putida, Aspergillus fumigatus, and Mucor circinelloides. Polymicrobial infection is common in forestry injuries, but we were surprised to reveal Mucor circinelloides in multiple probes of the ulna and flexor compartment. Concerning the rare and potentially fatal Mucor infection we had no specific treatment algorithm. Uncertainty persisted whether the Mucor species was angioinvasive until 15 days after amputation of the forearm owing to a decalcification process of bone samples by the pathology institute. Periodic acid-Schiff stain (PAS) and Grocott-Gomori’s methenamine-silver stain (GMS) were used in tissue sections. But for 15 days we had no conclusive information about its’ invasiveness. In accordance with the advice of our infectiologists, the antifungal therapy was escalated to Isavuconazol, and the antibiotic treatment to Tienam. Unfortunately, the patient developed acute renal and multi-organ failure within three days due to the aggressive antifungal therapy with isavuconazol. The patient had to be transferred to intensive care unit for further treatment.
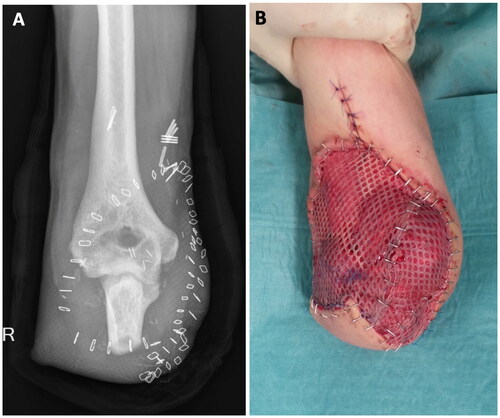
Only after we received histologic confirmation of colonization without angioinvasive infection of the latest debridement probes we could plan the onward therapy. From that point on treatment plan was changed from saving the patient’s life to preserving as much length of the ulna as possible to allow for a myoelectric prothesis. Eventually, closure of the skin defect by a fasciocutaneous hatchet flap and meshed skin graft took place 5 weeks after the initial trauma with a remaining ulnar stump length of 7 centimeters (). The graft healed well within three weeks. For further treatment including stump conditioning and adjusting a customized myoelectric prosthesis, the patient was transferred to a specialized rehabilitation center. Follow-up in our institution has been ongoing for over a year now without further complications.
Discussion
Mucormycosis is an exceptionally rare disease with a very high morbidity and mortality [Citation5]. The Mortality rate is reported to be 60 – 100% [Citation6]. Mucor are fungi that belong to the class of Zygomycetes that are usually present in our environment [Citation4–7]. Due to the rarity of the infection, it is almost impossible to conduct larger randomized clinical trials. A multidisciplinary approach is lifesaving as it often requires urgent medical interventions such as frequent surgical debridement and antifungal therapy [Citation5]. Many differing management regimes exist worldwide. Diagnosis of mucormycosis is challenging and an early diagnosis is of utmost importance [Citation1,Citation8]. Therefore a high grade of suspicion is required as the nature of the infection is rapidly progressive and destructive [Citation5]. Microscopy and culture are the mainstay of early diagnosis [Citation8]. Typical clinical findings are tissue necrosis from angioinvasion and subsequent thrombosis [Citation4,Citation9].
Additionally there are some conditions that predispose to Mucormycosis which include diabetes mellitus, hematologic malignancies, neutropenia, corticosteroids, and trauma among others [Citation2,Citation9]. Globally, diabetes is the leading underlying disease and the most important risk factor [Citation5,Citation9,Citation10]. A delay in proper infection treatment is associated with a higher mortality rate [Citation1,Citation5]. Jeong et al. reported a mortality rate of 46% and in patients with disseminated Mucormycosis even 68% [Citation10].
Most commonly fungal osteomyelitis is associated with Candida or Aspergillus species, but rarely with Mucormycosis [Citation1]. It is one of the most challenging complications in orthopedic and trauma surgery [Citation2]. Normally these infections are destructive and more virulent in immunocompromised patients [Citation2]. Osteoarticular Mucormycosis is progressive with soft tissue compromise and bone destruction that eventually may necessitate extremity amputation [Citation2]. In his study, Taj-Aldeen et al. reported that 56% of these infections were caused by direct inoculation in a trauma setting, 24% via hematogenous dissemination, and the remaining 21% consisted of a contiguous spread [Citation2]. Interestingly, they reported a median diagnostic delay of 60 days in all cases [Citation2]. Most of the patients in their cohort (85%) had a combination of antifungal therapy and surgery. Amphotericin B is the main antifungal treatment, but there are no controlled studies to support antifungal therapy in mucor-related osteomyelitis. Despite the effective treatment, Taj-Aldeen reported an overall mortality rate of 24% which is higher compared to other fungal osteomyelitis [Citation2].
In our patient we established antibiotic therapy according to the guidelines after replantation as we expected a polymicrobial infection. The origin of infection was direct inoculation with forestal flora. Unfortunately, multiple probes showed growth of Mucor circinelloides and we were uncertain for many days whether this was simply a colonization or a destructive and potentially lethal infection. Serial aggressive debridement, antibiotic, and antifungal therapy were performed to eradicate infection. Surgical treatment remained challenging as we intended to preserve as much of the proximal ulna and radius for future treatment with a myoelectric prosthesis. Eventually, cultures and biopsies were negative, and we closed the skin defect 5 weeks after the initial amputation with a fasciocutaneous hatchet flap and meshed skin graft. In this case, the exceptional challenge was that we identified the potentially fatal infection with Mucor species 7 days after replantation and uncertainty about its invasiveness persisted for 3 weeks after initial trauma. According to the guidelines the pathology institute used periodic acid-Schiff stain (PAS) and Grocott-Gomori’s methenamine-silver stain (GMS) in tissue section that eventually excluded tissue invasion [Citation5]. DNA samples weren’t used to differentiate between virulent and avirulent strains. To save the patient’s life, we decided to amputate the forearm. But overall, it remains unclear whether the detection of Mucor was a colonization from the beginning of the aggressive antifungal therapy prevented it from growing invasively. The crucial point is that retrospectively, it was a colonization of the remaining stump, we didn’t further test the amputation where the risk of invasion was probably higher. Therefore, it is of utmost importance to distinguish between a life-threatening angioinvasive infection and an ordinary colonization as soon as possible. In our case, a multidisciplinary approach was established on the day of trauma. But initially, we haven’t searched for fungal infections, and one could expect a polymicrobial inoculation with rare microbes in a forestry injury but also in battlefield injuries or immunocompromised patients.
Hyperbaric oxygen (HBO) has been used as an adjunctive treatment since the 1970s [Citation3]. Clinical data in the treatment of fungal infections with HBO is still limited and inconsistent. However according to more recent literature, it seems to be a useful tool in the multimodal treatment approach in this devastating infection [Citation3,Citation6].
Conclusion
As Mucormycosis infections are exceptionally rare and potentially lethal infections, an early multidisciplinary approach is mandatory. Patient’s with Mucor infection need treatment in a hospital with the highest care level. A high level of suspicion in patients with forestry injuries, war wounds or immunosuppressive conditions is reasonable. Early diagnosis, a combination of antifungal therapy with Amphotericin B and aggressive serial debridement as well as treatment of underlying medical conditions such as diabetes mellitus are mandatory. An immediate differentiation between colonization and invasiveness is crucial and can reduce side effects by shortening the time of treatment with antifungal agents as colonization can be treated with local antiseptics. Histopathologically, Mucor infection is confirmed by using PAS and GMS tissue sections. Most importantly, after forestry injuries, we advise prompt consultation with a department of infectious diseases as well as an institute of molecular pathology.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Tiourin E, Kanack M, Ng W, et al. Mucor osteomyelitis of the distal radius necessitating ulnocarpal fusion. Cureus. 2021;13(1):e12813. doi: 10.7759/cureus.12813.
- Taj-Aldeen SJ, Gamaletsou MN, Rammaert B, et al. Bone and joint infections caused by mucormycetes: a challenging osteoarticular mycosis of the twenty-first century. Med Mycol. 2017;55(7):691–704. doi: 10.1093/mmy/myw136.
- John BV, Chamilos G, Kontoyiannis DP. Hyperbaric oxygen as an adjunctive treatment for zygomycosis. Clin Microbiol Infect. 2005;11(7):515–517. doi: 10.1111/j.1469-0691.2005.01170.x.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54 Suppl 1(1):S23–S34. doi: 10.1093/cid/cir866.
- Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the european confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3.
- Segal E, Menhusen MJ, Simmons S. Hyperbaric oxygen in the treatment of invase fungal infections: a single-center experience. Isr Med Assoc J. 2007;9(5):355–357.
- Negi R, Kaushik R, Singh S, et al. Mucor as a cause of surgical site infection. Trop Doct. 2020;50(3):249–251. doi: 10.1177/0049475520921284.
- Skiada A, Lass-Floerl C, Klimko N, et al. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018;56(suppl_1):93–101. doi: 10.1093/mmy/myx101.
- Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi. 2020;6(4):265. doi: 10.3390/jof6040265.
- Jeong W, Keighley C, Wolfe R, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25(1):26–34. doi: 10.1016/j.cmi.2018.07.011.