ABSTRACT
Excessive positive energy balance is a major factor leading to obesity. The ability to alter the appetite-regulating hormones leptin, adiponectin, and ghrelin may help decrease excessive energy intake. Exercise and exposure to extreme temperatures can independently affect these appetite-regulating hormones. PURPOSE: To determine the effect of exercising in different environmental conditions on the circulating concentrations of leptin, adiponectin, and ghrelin. METHODS: Eleven recreationally-trained male participants completed 3 separate 1 h cycling bouts at 60% Wmax in hot, cold, and room temperature conditions (33°C, 7°C, 20°C), followed by a 3 h recovery at room temperature. Blood was drawn pre-exercise, post-exercise, and 3 h post-exercise. Hematocrit and hemoglobin were measured to account for change in plasma volume. RESULTS: Leptin concentrations were lower at post and 3 h post-exercise compared with pre-exercise, with and without correction for plasma volume shifts, regardless of temperature (p < 0.05). Adiponectin was higher post-exercise compared with pre-exercise (p = 0.021) but not 3 h post-exercise (p = 0.084) without correction for plasma volume shifts. However, adiponectin concentrations were not different at any time point when plasma volume shifts were accounted for (p > 0.05). Total ghrelin and acylated ghrelin concentrations were not affected at post and 3 h post-exercise compared with pre-exercise, with and without correcting for plasma volume shifts, regardless of ambient temperature (p > 0.05). No differences in leptin, adiponectin, or ghrelin were found between trials (p > 0.05). CONCLUSION: Temperature does not affect the circulating concentrations of appetite-regulating hormones during an acute bout of endurance exercise.
Introduction
Environmental temperature has a large impact on fat mass and metabolic homeostasis. Indigenous populations that live in polar climates have elevated basal metabolic rates and decreased fat mass,Citation1-3 whereas populations from tropical climates have decreased basal metabolic rates and increased fat mass.Citation4,5 Thus, there appears to be an effect of temperature on energy balance. However, the relationship between environmental temperature and the circulating concentration of appetite-regulating hormones is less clear. Exercise has been shown to be an effective method for altering the appetite-regulating hormones leptin, adiponectin, and ghrelin.Citation6-14 Exercise in a hot environment, compared with a room temperature environment appears to attenuate appetite.Citation15,16 However, exercise in a cold environment may stimulate appetite.Citation17
Adipose tissue plays an important role in the effect that different environmental temperatures have on appetite.Citation18 Adipose tissue can act as an endocrine organ, instead of solely as an energy storage depot.Citation19-22 Adipokines are secreted by adipose tissue and are involved in homeostatic and appetite-regulating signaling in the body. Specifically, leptinCitation23 and adiponectinCitation24 are adipokines that play a major role in energy homeostasis and appetite regulation.Citation25 Leptin signals the hypothalamus that energy requirements are being met and that no more food intake is required.Citation26 Adiponectin also acts at the hypothalamus but works to stimulate food intake.Citation27 Ghrelin is produced and released from the fundus of the stomach.Citation28 Unlike leptin, ghrelin is an orexigenic or an appetite-stimulating hormone.Citation29 Acylation of ghrelin is essential for appetite regulation.Citation30 The ability to alter these hormones could lead to a better control of appetite and potentially used in treating conditions commonly associated with increased levels of fat mass such as obesity, metabolic syndrome, and type 2 diabetes. Increasing leptin concentrations and decreasing adiponectin and ghrelin may lead to a decreased appetite. This change in hormone concentration could lead to a reduction in caloric intake and thus a more negative energy balance resulting in a subsequent reduction in fat mass.
Exercise is a well-known therapy to combat the conditions of obesity, metabolic syndrome, and diabetes.Citation31 Circulating concentrations of leptin generally decrease,Citation6-8,13,14 whereas adiponectin generally increases in response to an exercise bout.Citation9-12 It has been demonstrated that an acute exercise bout suppresses plasma levels of acylated ghrelin,Citation30 but has no effect on total ghrelin.Citation32 Thus, exercise induces an increased appetite in an attempt to replace energy reserves. However, there is contradicting evidence that claims exercise has no effect on these hormones.Citation6,7,33-35 The exact response of these hormones appears to be influenced by intensity, mode, and duration of exercise and may help explain the variability of response in these previous studies.
The ambient temperature may further alter adipokine response related to appetite. Research investigating the relationship between environmental temperature and the cytokines responsible for appetite regulation is limited to few human trials. These research studies show no change in circulating hormone levels. Current study investigates the influence of temperature in conjunction with endurance exercise on circulating hormone levels. To our knowledge, this is the first study attempting to investigate this relationship in a human model. Previously, results in human trials demonstrated conflicting results.Citation15,16,36 However, in a mouse model, heat exposure generally increased leptin and adiponectin concentration while cold exposure decreased leptin and adiponectin concentration.Citation37-40
Temperature and exercise both appear to independently affect leptin, adiponectin, and ghrelin. The potential impact of exercising in different environmental temperatures on appetite-regulating hormones is unknown. If there is an effect, however, it may lead to the development of temperature-optimized exercise protocols to control appetite. These temperature-optimized protocols could be implemented in the prevention and treatment of chronic conditions associated with excessive positive energy balance. Thus, the purpose of the current study is to determine the effects of exercise in hot, cold, and room temperature environmental conditions on circulating levels of leptin, adiponectin, and ghrelin in humans.
Materials and methods
Subjects
This study recruited 11 recreationally-trained male subjects (age 25 ± 4, height 178 ± 5 cm, weight 79.4 ± 13.5 kg, body fat 14.7 ± 3.6%, VO2peak 54.6 ± 12.0 ml· kg−1· min−1, power at VO2peak 277 ± 41 W). Recreationally-trained was defined as engaging in physical activity at least 1-2 times per week. Due to hormonal differences in men and women, exclusively male subjects were recruited for the study. Participants were between the ages of 19 and 45 and capable of cycling consistently for one hour. Participants were considered “low risk” according to ACSM risk stratification. All participants were to understand and sign an Institutional Review Board approved informed consent form before participating in this study.
Preliminary testing
The initial testing session included an exercise protocol to determine maximal aerobic capacity and collection of participant descriptive data. Specifically, height (Seca North America, Chino, CA), weight (Befour, Saukville, WI), body composition and VO2peak were assessed. Body composition was assessed using a hydrostatic weighing technique that uses a custom load cell based system (Exertech, Dresbach, MN). Body density was converted to percent body fat using the Siri equation.Citation41 To determine VO2peak participants performed a graded exercise test using an electronically braked cycle ergometer (Velotron, RacerMate Inc., Seattle WA). The test began at 95 W and workload was increased by 35 W every 3 min. Participants cycled until volitional fatigue and the highest VO2 obtained was recorded as the VO2peak. The maximum workload was measured by adding the highest completed stage (in watts) to the proportion of the last stage multiplied by the 35 W per stage increment. During the experimental trials participants cycled at 60% of this maximum calculated workload.
Experimental protocol
Each participant completed 3 separate trials that were no less than 4 and no more than 7 d apart. Average monthly temperature for the testing period (April–May) was 56.8°F. Participants tested were natives of the area, therefore not recently exposed to prolonged periods of hot or cold climates.
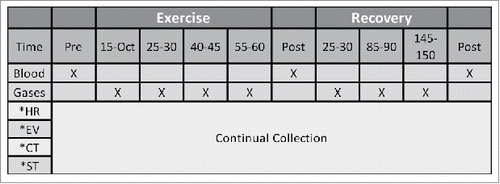
For each trial, the subject cycled in one of the experimental environmental conditions chosen by a randomized, counterbalanced, repeated measures design. The 3 trials consisted of a hot (33°C, 60% humidity), cold (7°C, 60% humidity), or room temperature (20°C, 60% humidity) environment. Participants came into the laboratory following a 12 h overnight fast and having not engaged in exercise within the previous 24 h. A 24 h food consumption log was maintained by the participant and replicated as closely as possible between trials. As subjects initially entered the laboratory, urine specific gravity was tested to ensure consistent hydration between trials (Pocket Refractometer, ATAGO Tokyo, Japan). Prior to exercise, a resting blood draw (∼6 mL) was taken from the antecubital vein of the arm. During the experimental portion of the trial, participants cycled at 60% of the peak aerobic power that was previously determined. The cycling bout was one hour in a temperature and humidity controlled environmental chamber (Darwin Chamber Company, St. Louis, MO). During the exercise bout, subjects were also required to ingest 500 mL of water. Immediately post-exercise, subjects were removed from the chamber, had blood drawn, and began a 3 h recovery period lying in a supine position quietly at room temperature (∼22°C). Participants were not allowed to eat during the recovery. At the end of the 3 h recovery period another blood draw was taken ().
Measurements
Core temperature, skin temperature, and heart rate
Immediately upon arrival for the experimental trials (∼45 min before exercise), subjects ingested a Jonah Core Body Temperature Capsule (JCBC, Hidalgo Limited, Cambridge, UK). After capsule ingestion, subjects drank 125 mL of water and ate a fiber bar (Fiber One bar, 140 kcal, 4 g fat, 29 g carbohydrates, 2 g protein) to facilitate movement of the capsule out of the stomach and into the small intestine. The capsule transmitted core temperature data and heart rate to an EQ02 LifeMonitor Sensor Electronics Module vest (SEM, Hidalgo Limited, Cambridge, UK) that was worn throughout the exercise and recovery.
Blood draws and plasma levels
Blood draws were taken before exercise, immediately post-exercise, and 3 h post-exercise from the antecubital vein of the arm. Approximately 6 mL of blood was drawn into an Ethylenediaminetetraacetic acid (EDTA) coated vacutainer, (Greiner Bio-One, Monroe, NC) and whole blood was immediately tested for hematocrit (ZIPOcrit, LW Scientific, Lawrenceville, GA) and hemoglobin (HemoCue Cypress, CA) for the calculation of plasma volume shifts that occurred throughout each trial.Citation42 The whole blood was then centrifuged at 10,000 X g for 15 min to separate plasma for later analysis. The plasma was aliquoted and stored at −80°C.
Circulating leptin, adiponectin, and ghrelin
Circulating plasma levels of the hormones of interest were quantified by enzyme-linked immunosorbent assays (ELISA). Protocols for each specific hormone were completed according to manufacturer's instructions. Prepackaged ELISA kits from Invitrogen (Life Technologies Corporation, Frederick, MD) were used to measure leptin and adiponectin. Prepackaged ELISA kits from Sigma-Aldrich (St. Louis, MO) were used to measure total ghrelin and kits from LifeSpan BioSciences Inc. (Seattle, WA) were used for acylated ghrelin. Plasma samples were diluted 1:100 for leptin, 1:2000 for adiponectin. No dilutions were used for ghrelin.
Statistical analysis
Environmental temperature, core and skin temperature, heart rate, VO2, and plasma content of each of the hormones of interest were analyzed with a repeated measures 2-way ANOVA (time x trial). If F-ratio values were found to be significant, a Fisher's protected LSD (least significant difference) post hoc was performed to evaluate where significance occurred. A probability of type I error of less than 5% was considered significant (p < 0.05). All statistical data were analyzed using the Statistical Package for Social Sciences software (SPSS 23.0; Chicago, IL). Data are reported as mean ± SE (standard error).
Results
Core and skin temperature
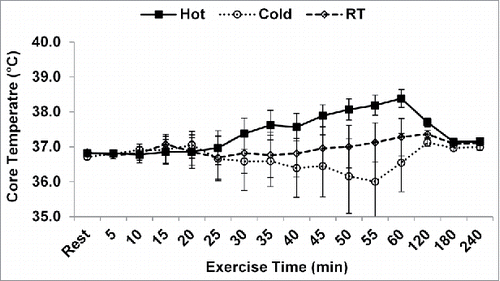
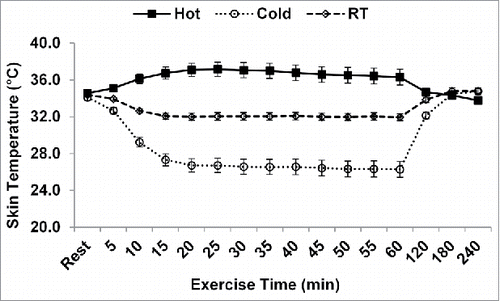
Core temperature was higher in the hot condition than the cold and room temperature conditions at 50, 55, and 60 min (p < 0.05; ). During recovery, core temperature was higher in the hot condition (37.4 ± 0.3°C) compared with the cold (37.1 ± 0.3°C, p = 0.001), and room temperature conditions (37.1 ± 0.2°C, p = 0.038), regardless of time. Skin temperature was highest in the hot condition and lower in the cold condition, compared with the room temperature condition by minute 5 of exercise and persisted to be different throughout the exercise bout (p < 0.001; ). During the recovery period, there were no differences in skin temperature between the 3 conditions (hot: 34.2 ± 1.0°C, cold: 33.6 ± 1.1°C, room temperature: 34.4 ± 0.8°C; p = 0.101).
Oxygen consumption
During exercise, both absolute and relative oxygen consumption were higher in the hot condition (3.0 ± 0.5 L· min−1, 38.5 ± 6.6 mL · kg−1 · min−1) and lower in the cold condition (2.7 ± 0.4 L· min−1, 34.5 ± 5.4 mL · kg−1 · min−1), compared with room temperature (2.8 ± 0.4 L· min−1, 35.7 ± 5.3 mL · kg−1 · min−1; p < 0.05). During recovery there were no differences in absolute (hot: 0.35 ± 0.05 L· min−1, cold: 0.33 ± 0.05 L· min−1, room temperature: 0.34 ± 0.05 L· min−1; p = 0.237), or relative oxygen consumption (hot: 4.5 ± 0.4 mL · kg−1 · min−1, cold: 4.3 ± 0.4 mL · kg−1 · min−1, room temperature: 4.3 ± 0.5 mL · kg−1 · min−1; p = 0.236).
Heart rate
During exercise, heart rate in the hot condition (165 ± 10 bpm) was higher than in the cold (152 ± 8 bpm) and room temperature condition (154 ± 11 bpm; p < 0.001), but no difference was observed between cold and room temperature (p = 0.318) conditions. During recovery, there were no differences in heart rate, but there was a trend toward higher heart rate in the hot condition (82 ± 11 bpm) compared with cold (75 ± 12 bpm) and room temperature (76 ± 10 bpm; p = 0.067) conditions.
Plasma leptin concentrations
Hematocrit and hemoglobin levels were significantly lower in the hot condition, compared with cold and room temperature conditions (p < 0.05).
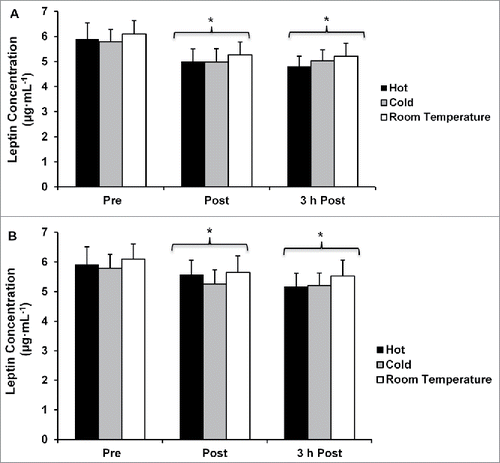
There were no differences in leptin concentrations between any of the temperature conditions when concentrations were not corrected for plasma volume shifts (p = 0.458; ), or when concentrations were corrected for plasma volume shifts (p = 0.465; ). However, leptin concentrations were lower at post-exercise and 3 h post-exercise when compared with pre-exercise regardless of temperature and with and without correcting for plasma volume shifts (p < 0.05, ).
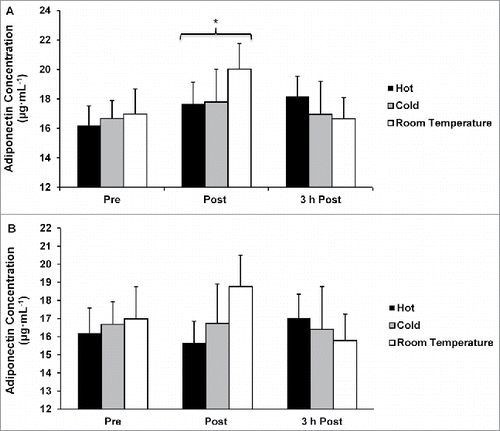
Plasma adiponectin concentrations
There were no differences in adiponectin concentrations between any of the temperature conditions without correcting concentration for plasma volume shifts (p = 0.752; ), or when concentrations were corrected for plasma shifts (p = 0.691; ). Adiponectin plasma concentrations were higher post- exercise when compared with pre-exercise when concentrations were not corrected for plasma volume shifts (p = 0.021; ). There were no differences between the pre-exercise and 3 h post-exercise adiponectin concentrations when uncorrected for plasma volume shifts (p = 0.395; ). When adiponectin concentration was corrected for plasma volume shifts, no differences between any of the time points were observed (p = 0.790; ).
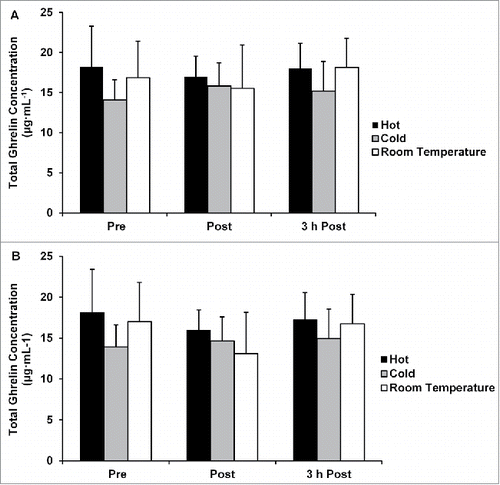
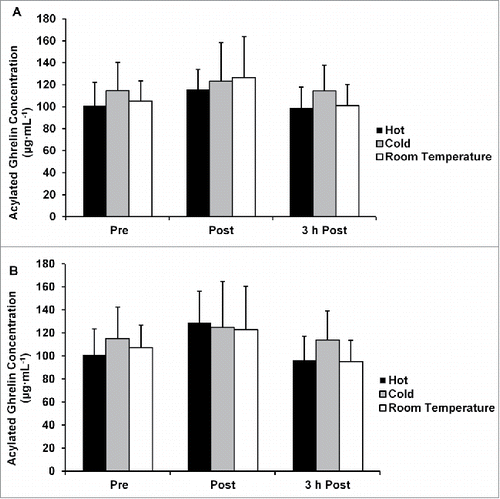
Plasma ghrelin concentrations
There were no differences in acylated and nonacylated ghrelin concentrations between any of the temperature conditions without correcting concentration for plasma volume shifts (p = 0.917 and p = 0.963; and ), or when concentrations were corrected for plasma shifts (p = 0.939 and 0.792; and ).
Discussion
This study proposed that exercising in different environmental temperatures would alter circulating concentrations of appetite-regulating hormones. Based on previous research, it was hypothesized that in a hot environment both leptin and adiponectin would increase compared with a room temperature condition. It was also hypothesized that in a cold environment both leptin and adiponectin would decrease compared with a room temperature environment. However, contrary to the hypothesis, the results of this study did not demonstrate temperature related effects on these hormones. Exercise independent of temperature did stimulate a decrease in leptin and a potential increase in adiponectin, but had no effect on circulating levels of total and acylated ghrelin. This hormonal profile would suggest an increase in appetite after exercise.
Research that has linked changes in leptin and adiponectin concentration to different temperature exposures has used mice and cell line models. Mice subjected to 8°C for 12 h had decreases in leptin concentrations.Citation39 Additionally, mouse preadipocytes exposed to 39°C and 41°C produced incremental increases in leptin when compared with cells exposed to 37°C.Citation43 Similar to leptin in a mouse model, mice that were exposed to chronic heat stress showed elevated adiponectin concentrations,Citation38 whereas, during a 24 h exposure to 4°C adiponectin levels were decreased.Citation37 It is difficult to interpret the effect on appetite when an appetite-reducing hormone (leptin) and an appetite-stimulating hormone (adiponectin) are increased or decreased together. However, in the case of temperature, leptin appears to play a more dominant role since exercise in the heat suppresses appetite,Citation15,16,36 while exercise in the cold increases appetiteCitation15,16 relative to a room temperature exercise condition. Based on this previous data showing an effect of temperature on hormones and appetite, we were surprised not to see any effect of temperature on leptin and adiponectin using our human exercise model. Furthermore, current findings may be attributed to less influence of temperature on thermoregulatory responses in humans, compared with rodents. Specifically, high body surface area-to-mass ratio and differences in metabolism and thermal tolerance of rats may explain changes in hormone responses compared with those observed in humans.Citation44,45
Lack of differences in hormone concentrations between temperatures in the current study may be due to the relatively short exposure times incorporated into the current design. In non-human models exposures were 8 to 24 times longerCitation37,38 and more intense,Citation37-39,43 than in the current study. Prolonged extreme temperature exposure may cause a greater difference in core body temperature compared with the moderate, short-lived effects observed here. A greater change in core body temperature may be required to induce the changes in hormone concentration that have been observed in non-human models. Ethical limitations using a human model may preclude this effect to be observed and thus may not have practical implications.
The current study did not directly measure appetite, and thus we cannot be certain that the lack of hormonal differences between exercise in different temperatures does not affect appetite. Indeed, previous research studies have shown a change in subjective appetite without a corresponding change in appetite-regulating hormones.Citation15,16,36 Further mechanistic work is needed to determine the sensitivity of hormonal concentration to predict appetite, as factors such as receptor number and receptor sensitivity may also be important.
Leptin and adiponectin are secreted from adipose tissue, and therefore leptin and adiponectin concentrations are elevated in obese individuals due to the increased adipose tissue mass. Much of the previous research examining appetite-regulating hormones has focused on overweight or obese populations.Citation7,8,46 The present study used recreationally active, relatively fit, and healthy male participants. These individuals were not having the hormonal imbalances, and/or receptor sensitivity issues associated with excess adipose tissue.Citation47,48 This population may be able to better regulate homeostasis when adding the additional stress of extreme temperatures and exercise.Citation48,49 During the hot trial heart rate and oxygen consumption were higher than the other trials. This physiologically more challenging trial likely placed additional stress on the participants. It appears that the current participants were able to adapt to this slight elevation in stress and relative intensity, whereas a less trained population may not be able to withstand these stresses without augmenting appetite-regulating hormones. Thus, it is possible that less trained individuals may show a different hormonal response to exercise in hot or cold environmental conditions than the current participants.
Results from the current study are in agreement with previous research that demonstrates decreases in leptin concentrations,Citation7,8,13,14,50 and increases in adiponectin concentrations with exercise.Citation9-12 Previous literature has reported that exercise bouts shorter than 60 min may not alter leptin concentration.Citation6 However, the current study using a 60 min cycling bout did result in decreased leptin levels. Not only are changes in leptin concentrations dependent on duration, but also depends upon intensity and exercise mode.Citation13,35,51-54 Thus, it appears that a 60 min bout of cycling at 60% of maximal workload associated with VO2max is sufficient to alter leptin and adiponectin concentrations in a recreationally trained healthy population. Furthermore, it has been demonstrated that swimming stimulates appetite and acylated ghrelin concentrations and lowers energy balance when compared with no exercise control.Citation55
Blood plasma volume lost during exercise by way of sweating and respiration should be accounted for when analyzing concentrations of circulating hormones. The loss of plasma will increase hormone concentration without a change in absolute hormone level. The current study analyzed the concentrations of leptin and adiponectin with and without considering changes due to plasma volume shifts.Citation42 Leptin was decreased post exercise when changes in plasma volume were not considered and when changes in plasma volume were considered. When adiponectin concentrations were uncorrected for plasma volume shifts, adiponectin increased post-exercise. Whereas, correcting for the plasma volume shifts resulted in no changes in adiponectin or ghrelin concentrations. It is uncertain whether an increased concentration of adiponectin due to a reduction in plasma volume is sufficient to cause a physiologic change or if an increase in total adiponectin is needed. No clear standard has been established on the need to correct for plasma volume shifts as there are many cases in which these shifts are ignored, calculated but not corrected for, or calculated and corrected for.Citation56 Thus, the current study reports both corrected and uncorrected concentrations so that future research may consider the impact of correcting leptin and adiponectin concentrations for shifts in plasma volume.
In conclusion, these results indicate that in healthy, recreationally-trained human participants, environmental temperature has no effect on the circulating concentrations of leptin, adiponectin or ghrelin immediately post-exercise or 3 h post-exercise. Furthermore, the current protocol seems to be of adequate duration, intensity, and mode to cause a decrease in leptin and increase of adiponectin concentrations. Further research is needed to determine the impact that temperature and exercise have on energy intake in the face of a change in leptin and adiponectin concentrations. Future studies investigating the effects of combined effects of exercise and temperature in clinical populations are warranted. Additionally, chronic exposure to temperature and exercise is needed to determine the therapeutic use of such interventions to treat and prevent conditions associated with high-energy intake.
Abbreviations
| ANOVA | = | analysis of variance |
| VO2peak | = | peak oxygen consumption |
| Wmax | = | Wattage max |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This publication was made possible by grants from the University Committee on Research and Creative Activity (UCRCA) and the National Institute for General Medical Science (NIGMS) (5P20GM103427), a component of the National Institutes of Health (NIH). The contents of this paper are the sole responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
References
- Leonard WR, Sorensen MV, Galloway VA, Spencer GJ, Mosher M, Osipova L, Spitsyn VA. Climatic influences on basal metabolic rates among circumpolar populations. Am J Hum Biol. 2002;14(5):609-620. PMID:12203815; doi:10.1002/ajhb.10072.
- Galloway VA, Leonard WR, Ivakine E. Basal metabolic adaptation of the evenki reindeer herders of central siberia. Am J Hum Biol. 2000;12(1):75-87. PMID:11534006; doi:10.1002/(SICI)1520-6300(200001/02)12:1%3c75::AID-AJHB9%3e3.0.CO;2-G.
- Rode A, Shephard RJ. Basal metabolic rate of inuit. Am J Hum Biol. 1995;7(6):723-729. doi:10.1002/ajhb.1310070607.
- Henry CJ, Rees DG. New predictive equations for the estimation of basal metabolic rate in tropical peoples. Eur J Clin Nutr. 1991;45(4):177-185. PMID:1824042.
- Soares MJ, Francis DG, Shetty PS. Predictive equations for basal metabolic rates of indian males. Eur J Clin Nutr. 1993;47(6):389-394. PMID:8365380.
- Kraemer RR, Chu H, Castracane VD. Leptin and exercise. Exp Biol Med (Maywood). 2002;227(9):701-708.
- Perusse L, Collier G, Gagnon J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Nadeau A, Zimmet PZ, Bouchard C. Acute and chronic effects of exercise on leptin levels in humans. J Appl Physiol (1985). 1997;83(1):5-10. PMID:9216937.
- Pasman WJ, Westerterp-Plantenga MS, Saris WH. The effect of exercise training on leptin levels in obese males. Am J Physiol. 1998;274(2 Pt 1):E280-E286. PMID:9486159.
- Ahmadizad S, Haghighi AH, Hamedinia MR. Effects of resistance versus endurance training on serum adiponectin and insulin resistance index. Eur J Endocrinol. 2007;157(5):625-631. PMID:17984242; doi:10.1530/EJE-07-0223.
- Kriketos AD, Gan SK, Poynten AM, Furler SM, Chisholm DJ, Campbell LV. Exercise increases adiponectin levels and insulin sensitivity in humans. Diabetes Care. 2004;27(2):629-630. PMID:14747265; doi:10.2337/diacare.27.2.629.
- Saunders TJ, Palombella A, McGuire KA, Janiszewski PM, Després J, Ross R. Acute exercise increases adiponectin levels in abdominally obese men. J Nutr Metab. 2012;2012:148729. PMID:22701167; doi:10.1155/2012/148729.
- Simpson KA, Singh MAF. Effects of exercise on adiponectin: A systematic review. Obesity. 2008;16(2):241-256. PMID:18239630; doi:10.1038/oby.2007.53.
- Durstine JL, Thompson RW, Drowatzky KL, Bartoli WP. Leptin and exercise: New directions. Br J Sports Med. 2001;35(1):3-4. PMID:11157452; doi:10.1136/bjsm.35.1.3.
- Dirlewanger M, Di Vetta V, Giusti V, Schneiter P, Jequier E, Tappy L. Effect of moderate physical activity on plasma leptin concentration in humans. Eur J Appl Physiol Occup Physiol. 1999;79(4):331-335. PMID:10090632; doi:10.1007/s004210050516.
- Kojima C, Sasaki H, Tsuchiya Y, Goto K. The influence of environmental temperature on appetite-related hormonal responses. J Physiol Anthropol. 2015;34(1):22. PMID:25935755; doi:10.1186/s40101-015-0059-1.
- Wasse LK, King JA, Stensel DJ, Sunderland C. Effect of ambient temperature during acute aerobic exercise on short-term appetite, energy intake, and plasma acylated ghrelin in recreationally active males. Appl Physiol Nutr Metab. 2013;38(8):905-909. PMID:23855280; doi:10.1139/apnm-2013-0008.
- White LJ, Dressendorfer RH, Holland E, McCoy SC, Ferguson MA. Increased caloric intake soon after exercise in cold water. Int J Sport Nutr Exerc Metab. 2005;15(1):38-47. PMID:15902988; doi:10.1123/ijsnem.15.1.38.
- Lenard NR, Berthoud H. Central and peripheral regulation of food intake and physical activity: Pathways and genes. Obesity. 2008;16(S3):S11-S22. PMID:19190620; doi:10.1038/oby.2008.511.
- Trayhurn P. Endocrine and signalling role of adipose tissue: New perspectives on fat. Acta Physiol Scand. 2005;184(4):285-293. PMID:16026420; doi:10.1111/j.1365-201X.2005.01468.x.
- Trayhurn P, Wood IS. Signalling role of adipose tissue: Adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33(Pt 5):1078-1081. PMID:16246049; doi:10.1042/BST0331078.
- Mohamed-Ali V, Pinkney J, Coppack S. Adipose tissue as an endocrine and paracrine organ. Int J Obes. 1998;22:1145-1158. PMID:9877249; doi:10.1038/sj.ijo.0800770.
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548-2556. PMID:15181022; doi:10.1210/jc.2004-0395.
- Luheshi GN, Gardner JD, Rushforth DA, Loudon AS, Rothwell NJ. Leptin actions on food intake and body temperature are mediated by IL-1. Proc Natl Acad Sci USA. 1999;96(12):7047-7052. PMID:10359836; doi:10.1073/pnas.96.12.7047.
- Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439-451. PMID:15897298; doi:10.1210/er.2005-0005.
- Fatouros I, Tournis S, Leontsini D, Jamurtas A, Sxina M, Thomakos P, Manousaki M, Douroudos I, Taxildaris K, Mitrakou A. Leptin and adiponectin responses in overweight inactive elderly following resistance training and detraining are intensity related. J Clin Endocrinol Metab. 2005;90(11):5970-5977. PMID:16091494; doi:10.1210/jc.2005-0261.
- Forbes S, Bui S, Robinson BR, Hochgeschwender U, Brennan MB. Integrated control of appetite and fat metabolism by the leptin-proopiomelanocortin pathway. Proc Natl Acad Sci USA. 2001;98(7):4233-4237. PMID:11259669; doi:10.1073/pnas.071054298.
- Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett. 2008;582(1):74-80. PMID:18054335; doi:10.1016/j.febslet.2007.11.070.
- Cummings DE. Ghrelin and the short-and long-term regulation of appetite and body weight. Physiol Behav. 2006;89(1):71-84. PMID:16859720; doi:10.1016/j.physbeh.2006.05.022.
- Meier U, Gressner AM. Endocrine regulation of energy metabolism: Review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50(9):1511-1525. PMID:15265818; doi:10.1373/clinchem.2004.032482.
- Broom DR, Stensel DJ, Bishop NC, Burns SF, Miyashita M. Exercise-induced suppression of acylated ghrelin in humans. J Appl Physiol (1985). 2007;102(6):2165-2171. PMID:17347386; doi:10.1152/japplphysiol.00759.2006.
- Church T. Exercise in obesity, metabolic syndrome, and diabetes. Prog Cardiovasc Dis. 2011;53(6):412-418. PMID:21545927; doi:10.1016/j.pcad.2011.03.013.
- Burns SF, Broom DR, Miyashita M, Mundy C, Stensel DJ. A single session of treadmill running has no effect on plasma total ghrelin concentrations. J Sports Sci. 2007;25(6):635-642. PMID:17454530; doi:10.1080/02640410600831856.
- Boudou P, Sobngwi E, Mauvais-Jarvis F, Vexiau P, Gautier JF. Absence of exercise-induced variations in adiponectin levels despite decreased abdominal adiposity and improved insulin sensitivity in type 2 diabetic men. Eur J Endocrinol. 2003;149(5):421-424. PMID:14585088; doi:10.1530/eje.0.1490421.
- Hulver MW, Zheng D, Tanner CJ, Houmard JA, Kraus WE, Slentz CA, Sinha MK, Pories WJ, MacDonald KG, Dohm GL. Adiponectin is not altered with exercise training despite enhanced insulin action. Am J Physiol Endocrinol Metab. 2002;283(4):E861-E865. PMID:12217905; doi:10.1152/ajpendo.00150.2002.
- Noland RC, Baker JT, Boudreau SR, Kobe RW, Tanner CJ, Hickner RC, McCammon MR, Houmard JA. Effect of intense training on plasma leptin in male and female swimmers. Med Sci Sports Exerc. 2001;33(2):227-231. PMID:11224810; doi:10.1097/00005768-200102000-00009.
- Shorten AL, Wallman KE, Guelfi KJ. Acute effect of environmental temperature during exercise on subsequent energy intake in active men. Am J Clin Nutr. 2009;90(5):1215-1221. PMID:19793848; doi:10.3945/ajcn.2009.28162.
- Morera P, Basirico L, Hosoda K, Bernabucci U. Chronic heat stress up-regulates leptin and adiponectin secretion and expression and improves leptin, adiponectin and insulin sensitivity in mice. J Mol Endocrinol. 2012;48(2):129-138. PMID:22279186; doi:10.1530/JME-11-0054.
- Imai J, Katagiri H, Yamada T, Ishigaki Y, Ogihara T, Uno K, Hasegawa Y, Gao J, Ishihara H, Sasano H. Cold exposure suppresses serum adiponectin levels through sympathetic nerve activation in mice. Obesity. 2006;14(7):1132-1141. PMID:16899794; doi:10.1038/oby.2006.130.
- Dutra SC, Moura EG, Rodrigues AL, Lisboa PC, Bonomo I, Toste FP, Passos MC. Cold exposure restores the decrease in leptin receptors (OB-rb) caused by neonatal leptin treatment in 30-day-old rats. J Endocrinol. 2007;195(2):351-358. PMID:17951546; doi:10.1677/JOE-07-0366.
- Song Z, Liu L, Sheikhahmadi A, Jiao H, Lin H. Effect of heat exposure on gene expression of feed intake regulatory peptides in laying hens. BioMed Res Int. 2012;2012:1-8.
- Siri WE. Body composition from fluid spaces and density: Analysis of methods. Tech Measuring Body Compos. 1961;61:223-244.
- Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37(2):247-248. PMID:4850854.
- Bernabucci U, Basirico L, Morera P, Lacetera N, Ronchi B, Nardone A. Heat shock modulates adipokines expression in 3T3-L1 adipocytes. J Mol Endocrinol. 2009;42(2):139-147. PMID:18996960; doi:10.1677/JME-08-0068.
- Gordon CJ. Thermal biology of the laboratory rat. Physiol Behav. 1990;47(5):963-991. PMID:2201986; doi:10.1016/0031-9384(90)90025-Y.
- Wanner SP, Prímola-Gomes TN, Pires W, Guimarães JB, Hudson ASR, Kunstetter AC, Fonseca CG, Drummond LR, Damasceno WC, Teixeira-Coelho F. Thermoregulatory responses in exercising rats: Methodological aspects and relevance to human physiology. Temperature. 2015;2(4):457-475. PMID:27227066; doi:10.1080/23328940.2015.1119615.
- Nindl BC, Kraemer WJ, Arciero PJ, Samatallee N, Leone CD, Mayo MF, Hafeman DL. Leptin concentrations experience a delayed reduction after resistance exercise in men. Med Sci Sports Exerc. 2002;34(4):608-613. PMID:11932568.
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19(2):155-157. PMID:9620771; doi:10.1038/509.
- Myers MG, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: Distinguishing cause from effect. Trends Endocrinol Metab. 2010;21(11):643-651. PMID:20846876; doi:10.1016/j.tem.2010.08.002.
- Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9(1):35-51. PMID:19117545; doi:10.1016/j.cmet.2008.12.004.
- Kraemer R, Durand R, Hollander D, Tryniecki J, Hebert E, Castracane V. Ghrelin and other glucoregulatory hormone responses to eccentric and concentric muscle contractions. Endocrine. 2004;24(1):93-98. PMID:15249708; doi:10.1385/ENDO:24:1:093.
- Kawano H, Mineta M, Asaka M, Miyashita M, Numao S, Gando Y, Ando T, Sakamoto S, Higuchi M. Effects of different modes of exercise on appetite and appetite-regulating hormones. Appetite. 2013;66:26-33. PMID:23402716; doi:10.1016/j.appet.2013.01.017.
- Olive JL, Miller GD. Differential effects of maximal-and moderate-intensity runs on plasma leptin in healthy trained subjects. Nutrition. 2001;17(5):365-369. PMID:11377127; doi:10.1016/S0899-9007(01)00522-6.
- Thompson DA, Wolfe LA, Eikelboom R. Acute effects of exercise intensity on appetite in young men. Med Sci Sports Exerc. 1988;20(3):222-227. PMID:3386499; doi:10.1249/00005768-198806000-00002.
- Ueda SY, Yoshikawa T, Katsura Y, Usui T, Fujimoto S. Comparable effects of moderate intensity exercise on changes in anorectic gut hormone levels and energy intake to high intensity exercise. J Endocrinol. 2009;203(3):357-364. PMID:19737911; doi:10.1677/JOE-09-0190.
- King JA, Wasse LK, Stensel DJ. The acute effects of swimming on appetite, food intake, and plasma acylated ghrelin. J Obes. 2011;2011. Epub 2010 Oct 3; PMID:20953411; doi:10.1155/2011/351628.
- Kargotich S, Goodman C, Keast D, Morton AR. The influence of exercise-induced plasma volume changes on the interpretation of biochemical parameters used for monitoring exercise, training and sport. Sports Med. 1998;26(2):101-117. PMID:9777683; doi:10.2165/00007256-199826020-00004.