 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Aging is associated with impairments in thermoregulatory function, which may augment the neuroendocrine and immune response in older relative to young adults during physical activity in the heat. This study was therefore aimed at examining changes in circulating endocrine hormones as cortisol (COR), prolactin (PRL), human growth hormone (hGH) and interleukin-6 (IL-6) in young and older men prior to and following an incremental, exercise-heat stress protocol (40°C and ~15% relative humidity). Accordingly, ten habitually active young (mean±SD; 21 ± 1 years) and ten older (65 ± 3 years) men performed three 30-min bouts of cycling at increasing metabolic heat productions (300, 400 and 500 W, equal to light, moderate and vigorous exercise), each separated by a 15-min recovery. Consistent with our hypothesis, we observed augmented IL-6 in older (3.55 ± 1.62 pg/mL) compared to young men (1.59 ± 0.88 pg/mL) following the protocol (p < 0.001). However, no significant between-group differences were observed for COR and hGH (all p > 0.050). We show that when assessed following incremental exercise in the heat, older men display augmented interleukin-6, but similar levels of stress hormones relative to young men.
Introduction
Physical activity in hot environments can cause increases in heat strain, which trigger the release of neuroendocrine hormones (e.g. cortisol [COR], human growth hormone [hGH], prolactin [PRL]) and cytokines (e.g. interleukin-6 [IL-6]) to maintain glucose homeostasis and to integrate the functions of different immune cells involved in tissue protection and repair [Citation1,Citation2]. These neuroendocrine and immune alterations may be considered as surrogate markers of the human physiological response to heat stress [Citation1,Citation3,Citation4]. However, to date, previous studies examining the stress hormones and the immunological responses to exercise in the heat are sparse and have primarily been limited to young males [Citation1]. Consequently, our understanding of age-related changes in these responses remains relatively poor, despite our warming climate and the rising demand for older adults to be engaged in physical activity for healthy aging.
Older individuals have a reduced capacity for heat dissipation in response to exercise-heat stress and accordingly experience greater body heat storage compared to young adults [Citation5]. However, it remained uncertain whether those age-related impairments worsened with increases in exercise intensity. As an extension of this work, Stapleton et al. [Citation6] therefore examined the extent to which aging modulates whole-body evaporative heat loss (HE) using a direct air calorimeter (a device that precisely measures whole-body heat exchange) during increasing levels of exercise-heat stress. Ten habitually active young (mean±SD; 21 ± 1 years) and ten older (65 ± 3 years) men performed three 30-min bouts of cycling at increasing metabolic heat productions (300, 400 and 500 W, equal to light, moderate and vigorous exercise), each separated by a 15-min recovery in the heat (40°C and ~15% relative humidity). Older men demonstrated a significant reduction in HE compared to young men during moderate (424 ± 38 vs 472 ± 42 W) and vigorous (485 ± 44 vs 558 ± 51 W) exercise, which exacerbated body heat storage and core (esophageal) temperature relative to young adults.
Given that aging is also associated with immune dysregulation that can include an altered neuroendocrine response [Citation4], it is plausible that these age-related reductions in thermoregulatory function may be coupled by an augmented neuroendocrine and immune response relative to young adults during physical activity in the heat. We therefore sought to examine changes in circulating COR, PRL, hGH and IL-6 in young and older men prior to and following the incremental, exercise-heat stress protocol employed by Stapleton et al. [Citation6]. We hypothesized that older men would demonstrate augmented cytokine and hormonal release compared to their younger counterparts.
Material and methods
This study was part of a larger investigation evaluating the effects of age and training status on thermoregulatory function, the experimental procedures of which are published elsewhere [Citation6]. These procedures are summarized below, with cross-references provided for the provision of additional detail.
The experimental protocol was approved by the University of Ottawa, Health Sciences and Science Research Ethics Board and is in agreement with the Declaration of Helsinki. All volunteers provided written and informed consent prior to their participation.
Ten young and ten older healthy, nonsmoking males, with similar physical characteristics participated (). Participants completed one preliminary and one experimental session each separated ≥48 hours. For both sessions, participants were instructed to arrive at the laboratory adequately rested and hydrated, having abstained from exercise, caffeine and alcohol 24 hours prior, and having eaten a standardized light meal (dry toast, orange juice) 2 hours prior.
Table 1. Participant characteristics
During the preliminary session, standing height, body mass, body density and peak aerobic power (V̇O2peak) were measured. Height and body mass were measured using an eye-level stadiometer (Detecto, model 2391, Webb City, MO, USA) and a high-performance digital weighing terminal (model CBU150X, Mettler Toledo Inc., Mississauga, ON, Canada), respectively, and used to estimate body surface area [Citation7]. Body fat percentage was calculated from body density [Citation8], which was determined using the hydrostatic weighing technique. V̇O2peak was determined during an incremental semi-recumbent cycling exercise test [Citation9].
Upon arrival for the experimental session, participants changed into athletic shorts and sandals before providing a urine sample to confirm euhydration (urine specific gravity: <1.025) [Citation10]. Following ~30 min instrumentation in a temperate room (~23ºC), participants sat in the semi-recumbent position within a direct air calorimeter regulated at 40°C and ~15% relative humidity. Participants then completed 30-min rest followed by three, 30-min bouts of cycling at increasing metabolic heat productions (Ex1: 300 W; Ex2: 400 W; Ex3: 500 W), each followed by a 15-min recovery. The rates of metabolic heat production for each exercise bout (Ex1, Ex2, Ex3) were equivalent to ~25, 35 and 45% V̇O2peak for the young and ~33, 47 and 60% V̇O2peak for the older group.
Metabolic heat production (metabolic rate – external work) was determined using indirect calorimetry (Moxus modular metabolic system, AEI Technologies, Pittsburgh, PA). Pre-trial urine specific gravity was assessed using a hand-held refractometer (model TS400; Reichter, Depew, NY, USA). Venous blood was collected at the end of the ~30-min seated instrumentation period prior to entering the calorimeter (PRE) and following the final 15-min resting recovery (POST) while participants remained seated inside the calorimeter via a single venipuncture (Becton, Dickinson and Company [BD], Franklin Lakes, NJ, USA) and then transferred directly into non-additive and K2EDTA 5.4 mg BD Vacutainer® tubes (BD, Franklin Lakes, NJ, USA). Non-additive blood sat for 20 min to clot before centrifugation at ~3,300 rpm (RCF 1,380 × g) for 10 min, whereas the K2EDTA blood was mixed by inversion, used to measure hematological parameters (i.e. hemoglobin, hematocrit, platelet count, and mean platelet volume) (Beckman Coulter, Miami, FL, USA) and centrifuged immediately. Blood hemoglobin and hematocrit values were used to estimate the percent changes in plasma volume [Citation11]. Following centrifugation, serum and plasma aliquots were transferred into polypropylene Eppendorf™ tubes, frozen at −20°C, and stored at −70°C until analysis. Enzyme immunoassay techniques were used to determine circulating serum IL-6, hGH, PRL, and COR (Quantikine® High Sensitivity R & D Systems, Minneapolis, MN) levels. The assays had minimum detectable concentrations (sensitivities) of 0.016–0.110 pg/mL, 0.5 ng/mL, 2.0 ng/mL, and 0.4 ug/Dl for IL-6, Hgh, PRL, and COR, respectively. All samples were analyzed in duplicate following manufacturer instructions and corrected for changes in plasma volume. Due to technical difficulties in some trials and during analysis, a reduced sample size is reported for young (n = 9) and older adults (n = 7) for these data.
Data were analyzed using a two-way, mixed-model ANOVA with factors of group (young, older) and time (pre, post). In the event of significant interaction or main effect, post-hoc comparisons were carried out using unpaired (group) or paired (time) two-tailed t-tests (α = 0.05 for all comparisons). Data are reported as means (±SD), unless stated otherwise as 95% CI. All analyses were performed using Prism version 8 (GraphPad Software, La Jolla, California, USA).
Results
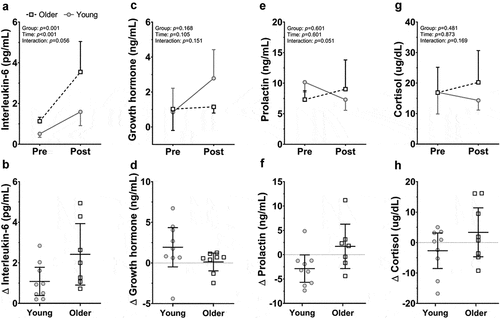
The change in plasma volume following the protocol was similar between groups (p = 0.353), averaging −8.8 ± 2.0% and −10.5 ± 2.9% for the young and older groups, respectively. Consistent with our hypothesis, IL-6 concentrations were similar between young and older men at rest, but greater in older compared to young men following the incremental exercise-heat stress protocol (p < 0.001) ()). No significant between-group differences in pre-exposure hGH, PRL, and COR were observed (all p > 0.184) (). Following the exposure, absolute concentrations of hGH and COR were similar prior to and following the exposure and did not differ significantly between groups (all p > 0.050) (). Finally, no significant between-group differences were observed for the relative changes in IL-6, hGH, PRL, and COR following the exposure (all p > 0.050) ().
Figure 1. Absolute and relative changes in concentrations of Interleukin-6 (a, b), Growth Hormone (c, d), Prolactin (d, e), and Cortisol (e, f) for the young and older groups prior to (PRE) and following (POST) the protocol. Data are means (± 95% CI) with individual data for both the young (solid circles and lines) and older groups (open squares and dashed lines). P values denote the results from a mixed model ANOVA comparing the effects of group (Young, Older) and time (PRE, POST)
Discussion
When assessed in young and older individuals after incremental intermittent exercise in dry heat, older men demonstrated altered stress hormone and IL-6 responses to exercise under heat. Precisely, we found that compared to their young counterparts, older men showed an augmented IL-6 response and tended to display an elevated PRL concentration after intermittent exercise in the heat. Although aging is associated with immune dysregulation that can result in elevated resting levels of circulating inflammatory markers [Citation4], the similar response of older and young men at rest may be ascribed to their regular engagement in physical activity (VO2peak: young 50.0 ± 3.7 ml/kg/min, older 37.9 ± 7.4 ml/kg/min), which can positively impact immune function [Citation12].
It has been demonstrated that IL-6 as a myokine is released due to a higher muscle sympathoadrenal and temperature response that hastens glycogenolysis [Citation13,Citation14]. Since aging is associated with skeletal muscle metabolism dysfunction due to changes in muscle function and structure [Citation15], the increased IL-6 concentration observed in this study was likely exacerbated due to augmented muscle glycogen use. Another possibility is that the elevated level of heat strain created by the impairment in heat dissipation during exercise may have triggered this heightened inflammatory response in older compared to young adults following the protocol [Citation4].
In line with the increase in IL-6 response, older men tended to have a greater elevation in PRL concentration after exercise in the heat, although this did not reach statistical significance ()). It is well-known that PRL release is related to changes in core temperature and heat stress [Citation3], as even a modest increase of 0.25°C in rectal temperature is capable of triggering PRL release [Citation16], and may be an indirect marker of central fatigue during exercise in the heat [Citation17]. It is possible, therefore, that the observed elevations in heat strain in older relative to young adults may too augment PRL secretion [Citation18], and this represents an important area of future research.
Despite these age-related increases in circulating IL-6 and PRL, COR and hGH did not differ significantly over time or between groups (all p > 0.050) (). Moreover, the secretion of COR and hGH during exercise-heat stress is largely observed at core temperatures >38°C [Citation1]. In this study, however, core temperature averaged ~37.4°C and ~37.9°C (~0.7°C vs. ~1.0°C when expressed as a change from baseline) for young and older men, respectively [Citation6]. It is plausible, therefore, that the level of thermal strain was insufficient to induce measurable increases in these stress hormones.
The world has entered uncharted territory as it experiences record high temperatures. It is an ominous signal of what increasing temperatures portend for vulnerable older adults who must endure these temperature extremes when performing physical activity or work in the heat. With the growing aging population and workforce, it is important to continue building our understanding of the factors involved in the age-related deterioration in thermoregulatory function. Given the increasing evidence that the human heat stress response is influenced by neuroendocrine and inflammatory factors, further research is needed to elucidate how these factors may be modulated by age-related differences in heat dissipation.
Author contributions
GPK conceptualized and designed the research. SRN analyzed data and prepared figures. All authors interpreted experimental results. ΑΚ drafted manuscript. All authors edited and revised the manuscript. All authors approved the final version.
Abbreviations
| COR | = | cortisol |
| hGH | = | human growth hormone |
| IL-6 | = | interleukin-6 |
| PRL | = | prolactin |
| HE | = | whole-body evaporative heat loss |
Acknowledgments
We would like to thank Dr. Heather Wright Beatty for assistance with data collection and analysis. This study was funded by the Canadian Institutes of Health Research (grant #399434).
Disclosure statement
No conflict of interest, financial or otherwise, is declared by the author(s). The results of the current study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Additional information
Funding
References
- Brenner IK, Zamecnik J, Shek PN, et al. The impact of heat exposure and repeated exercise on circulating stress hormones. Eur J Appl Physiol Occup Physiol. 1997;76(5):445–454.
- Mündel T, Cox J, Jones D. Exercise, heat stress and the interleukin-6 response: support for temperature-mediated neuroendocrine regulatory mechanisms. Medicina Sportiva. 2010;14(3):96–102.
- Wright HE, McLellan TM, Friesen BJ, et al. Influence of circulating cytokines on prolactin during slow vs. fast exertional heat stress followed by active or passive recovery. J Appl Physiol (1985). 2012;113(4):574–583.
- Wright HE, McLellan TM, Larose J, et al. Are circulating cytokine responses to exercise in the heat augmented in older men? Appl Physiol Nutr Metab. 2014;39(2):117–123.
- Stapleton JM, Larose J, Simpson C, et al. Do older adults experience greater thermal strain during heat waves? Appl Physiol Nutr Metab. 2014;39(3):292–298.
- Stapleton JM, Poirier MP, Flouris AD, et al. Aging impairs heat loss, but when does it matter? J Appl Physiol (1985). 2015;118(3):299–309.
- Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Nutrition. 1989;5(5):303–311. discussion 312–303.
- Siri WE. The gross composition of the body. Adv Biol Med Phys. 1956;4(239–279):513.
- Canadian Society for Exercise Physiology. Determination of aerobic power. In: Certified fitness appraiser resource manual. Gloucester, Ontario, Canada: Canadian Society for Exercise Physiology; 1986. p. 1–32.
- Kenefick RW, Cheuvront SN. Hydration for recreational sport and physical activity. Nutr Rev. 2012;70(Suppl 2):S137–142.
- Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37(2):247–248.
- Stewart LK, Flynn MG, Campbell WW, et al. The influence of exercise training on inflammatory cytokines and C-reactive protein. Med Sci Sports Exerc. 2007;39(10):1714–1719.
- Febbraio MA, Snow RJ, Stathis CG, et al. Blunting the rise in body temperature reduces muscle glycogenolysis during exercise in humans. Exp Physiol. 1996;81(4):685–693.
- Febbraio MA, Snow RJ, Hargreaves M, et al. Muscle metabolism during exercise and heat stress in trained men: effect of acclimation. J Appl Physiol (1985). 1994;76(2):589–597.
- Gheller BJ, Riddle ES, Lem MR, et al. Understanding age-related changes in skeletal muscle metabolism: differences between females and males. Annu Rev Nutr. 2016;36:129–156.
- Mündel T, Hooper PL, Bunn SJ, et al. The effects of face cooling on the prolactin response and subjective comfort during moderate passive heating in humans. Exp Physiol. 2006;91(6):1007–1014.
- Pitsiladis YP, Strachan AT, Davidson I, et al. Hyperprolactinaemia during prolonged exercise in the heat: evidence for a centrally mediated component of fatigue in trained cyclists. Exp Physiol. 2002;87(2):215–226.
- Brisson GR, Audet A, Ledoux M, et al. Exercise-induced blood prolactin variations in trained adult males: a thermic stress more than an osmotic stress. Horm Res. 1986;23(4):200–206.
