Comment on: Aldea D, Atsuta Y, Kokalari B, Schaffner SF, Prasasya RD, Aharoni A, Dingwall HL, Warder B, Kamberov YG. Repeated mutation of a developmental enhancer contributed to human thermoregulatory evolution. Proc Natl Acad Sci U S A. 2021 Apr 20;118(16):e2021722118. doi: 10.1073/pnas.2021722118. PMID: 33850016; PMCID: PMC8072367.
Humans cool themselves primarily through the evaporation of water from the skin surface. Human eccrine sweat glands, which are responsible of secreting the water for evaporative cooling, are essential for this mechanism of thermoregulation [Citation1]. Among primates, humans have the highest eccrine sweat gland density. Indeed, human eccrine sweat gland density is on average ten times that of chimpanzees and macaques [Citation2]. The high density of eccrine glands in human skin is one of the most dramatic phenotypic differences that distinguishes humans from other primates, and is a key component that makes possible humans’ exceptional ability to cool off by sweating [Citation1]. In spite of its importance, the genetic mechanisms behind the evolution of this adaptative human trait have been poorly studied.
In our recent study [Citation3], we identified the first genetic and developmental mechanism responsible for the evolutionary elaboration of human eccrine glands. Our study built on previous work from us and others showing that the upregulation of the transcription factor Engrailed 1 (En1) promotes the specification of eccrine glands in mice, and the upregulation of EN1 expression is correlated with eccrine gland development in humans [Citation4,Citation5]. We therefore sought to determine if humans have evolved regulatory mutations that lead to a potentiation of EN1 expression in the skin to induce the formation of more eccrine glands.
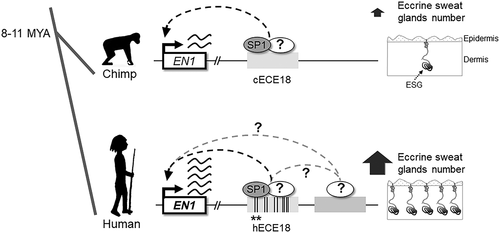
Since the spatial pattern of En1 expression is conserved across mammals in regions where eccrine glands form [Citation4–6], we first scanned the non-coding genome near the human EN1 locus for evolutionarily conserved sequences with the potential to act as regulatory elements, or enhancers, of this gene. To this end we utilized comparative genomics coupled with functional testing in transgenic mice to look for EN1 candidate enhancers (ECEs) that showed activity in En1-expressing skin cells during development. We identified 5 ECEs that exhibited such developmental enhancer activity in mouse eccrine forming skin. The human homolog of one positive ECE, which we named human ECE18 (hECE18) was the most highly sequence diverged relative to other apes. hECE18 was also notable in that it overlapped 2xHAR20, one of a class of computationally-defined genomic elements characterized by high sequence conservation across non-human vertebrates but exceptionally high divergence in humans that have been postulated to play roles in generating human-specific evolutionary phenotypes [Citation7]. In addition, we found that hECE18 has the greatest enhancer activity in cultured human or mouse skin cells as compared to other mammalian ECE18 homologs. The higher activity of hECE18 is the result of the accumulation of multiple single nucleotide substitutions that have specifically evolved on the human lineage, and because two of these human mutations give a high-affinity binding to the SP1 protein when compared to other primates.
These results prompted us to test whether the hECE18 enhancer can affect the number of eccrine glands through the regulation of En1 in a developmental model. To that end we replaced the endogenous ECE18 sequence in the mouse genome with the homologous hECE18 sequence using CRISPR-Cas9 genome editing. Remarkably, we found that the human enhancer upregulates En1 expression in the skin to promote the specification of more eccrine glands during skin development. Coupled with our findings of increased hECE18 activity in vitro, our data from the developmental mouse model are consistent with an evolutionary scenario where by the repeated mutation of the single En1 enhancer led to successive gains in ectodermal EN1 expression specifically on the human lineage to drive the specification of more eccrine glands in human skin as compared to that of other primates ().
Figure 1. En1 sweat we trust: How the evolution of an Engrailed 1 enhancer made humans the sweatiest ape. Over the course of the human evolution the human ECE18 enhancer accumulated mutations (highlighted as vertical black bars) leading to increased Engrailed 1 expression in human skin to induce the specification of more eccrine sweat glands (ESG) relative to other primates. The transcription factor SP1 is one of the key factors that binds the ECE18 enhancer. Two human derived sites (denoted with asterisks) reside within conserved SP1 motifs, these human mutations give a high-affinity binding to the SP1 protein when compared to other primates. Ongoing studies are pursuing the identity of cognate factors whose association with hECE18 was changed by the evolution of the remaining mutations within the human enhancer. Future studies will also focus on the characterization of the remaining positive En1 candidate enhancers reported in our study. What factors binds these elements and how they interact with each other will be the central focus of our investigation with implications to human evolution, development and translational medicine.
ECE18 is to date the first known EN1 vertebrate enhancer. Learning about such regulatory regions and molecular factors that specify and pattern eccrine sweat glands will contribute to the generation of new tissue engineering technologies with important clinical implications for regenerating eccrine glands. Thus, our study provides a genetic foundation for investigating the broader eccrine gland developmental program, paving the way to discover the suite of molecular factors and regulatory non-coding sequences that control En1 expression during eccrine sweat gland formation. Accordingly, future work to identify the transcription factors that bind and regulate the hECE18 enhancer, and examining the functional role in EN1 regulation and cognate trans-acting factors that control the other positive ECEs identified by our study will have direct implications for uncovering the program for patterning and specification of eccrine glands.
Teasing apart the suite of skin-specific EN1 enhancers provides an opportunity to study the EN1 locus at a structural chromatin level. Therefore, studying the different promoter-enhancers or enhancer-enhancer interactions and the proteins involved in the regulation of EN1 expression will help us to predict the impact of polymorphisms on the natural variation of eccrine sweat glands number among human populations or in other species. This is particularly important since direct interrogation of human eccrine gland density is confounded by the invasive methods needed to accurately quantify anatomical eccrine glands in our species. Moreover, from an evolutionary perspective, by setting the stage for parsing out how the different ECE18 mutations were fixed during human evolution, our work lays the foundation for understanding the timing and phenotypic increments of the evolution of human thermoregulatory capabilities. Additionally, given that EN1 expression persists in adult eccrine glands through-out life, our findings raise the possibility that the evolution of the human EN1 enhancer may also have additional implications for human thermoregulation beyond its effects in promoting eccrine gland formation. Future studies to address the potential contribution of EN1 and of the derived changes in the hECE18 enhancer on the functionality and relative output of mature eccrine glands are needed to determine if hECE18 could have played such additional roles in differentiating humans’ extreme capacity for evaporative cooling from the skin.
Humans are facing one of the worst climate emergencies in history and global temperatures are rising. The human thermoregulatory system is being increasingly taxed by unprecedented extremes of heat and humidity. It is therefore crucial to understand the genetic pathways and factors that promote and control the formation of eccrine sweat glands. Our study [Citation3] reveals a genetic foundation for humans’ exceptional sweating capabilities, and provides a platform for future investigation. Thus, now more than ever, while the times ahead seem challenging, we can rely on our remarkable and unique sweating capabilities and say: “En1 sweat we trust”.
References
- Kuno Y, Human perspiration. Springfield, Ill.: Thomas, 1956. [ Online]. Available: http://catalog.hathitrust.org/api/volumes/oclc/1100428.html
- Kamberov YG, Guhan SM, DeMarchis A, et al. Comparative evidence for the independent evolution of hair and sweat gland traits in primates. J Human Evol. 2018;125:99–105.
- Aldea D, Atsuta Y, Kokalari B, et al. Repeated mutation of a developmental enhancer contributed to human thermoregulatory evolution. Proc Nat Acad Sci. 2021;118(16):e2021722118.
- Kamberov YG, Karlsson EK, Kamberova GL, et al. A genetic basis of variation in eccrine sweat gland and hair follicle density. Proc Nat Acad Sci. 2015;112(32):9932–9937.
- Lu CP, Polak L, Keyes BE, et al. Spatiotemporal antagonism in mesenchymal-epithelial signaling in sweat versus hair fate decision. Science. 2016;354(6319):aah6102.
- Prin F, Logan C, D’Souza D, et al. Dorsal versus ventral scales and the dorsoventral patterning of chick foot epidermis. Dev Dyn Off Publ Am Assoc Anat. 2004;229(3):564–578.
- Pollard KS, Salama SR, King B, et al. Forces shaping the fastest evolving regions in the human genome. PLoS Genetics. 2006;2(10):e168.
