ABSTRACT
Thermoregulation is critical in health and disease and is tightly controlled to maintain body temperature homeostasis. Carbon monoxide (CO), an endogenous gasotransmitter produced during heme degradation by heme oxygenases, has been suggested to play a role in body core temperature (Tb) regulation. However, a direct involvement of CO in thermoregulation has not been confirmed and its mechanism(s) of action remain largely unknown. In the present study we characterized the effects of systemic delivery of CO by administration of an orally active CO-releasing molecule (CORM-401) on Tb regulation in conscious freely moving rats. Specifically, we evaluated the main thermo effectors in rats treated with CORM-401 by assessing: (i) non-shivering thermogenesis, i.e. the increased metabolism of brown fat measured through oxygen consumption and (ii) the rate of heat loss from the tail through calculations of heat loss index. We found that oral administration of CORM-401 (30 mg/kg) resulted in augmented CO delivery into the blood circulation as evidenced a by significant increase in carbon monoxy hemoglobin levels(COHb). In addition, treatment with CORM-401 increased Tb, which was caused by an elevated non-shivering thermogenesis indicated by increased oxygen consumption without significant changes in the tail heat loss. On the other hand, CORM-401 did not affect blood pressure, but significantly decreased heart rate. In summary, the findings of the present study reveal that increased circulating CO levels lead to a rise in Tb, which could have important implications in the emerging role of CO in the modulation of energetic metabolism.
Introduction
Carbon monoxide (CO), a gaseous molecule continuously produced during heme catabolism by the enzymes heme oxygenases (HO-1 and HO-2), plays a critical role as intracellular mediator and regulator of a variety of physiological functions. Indeed, CO serves as a neurotransmitter and exerts important vasodilatory, anti-ischemic and anti-inflammatory actions to the extent that therapeutic applications based on CO delivery have been under intense scrutiny [Citation1–4]. The physiological effects of CO have been partially attributed to CO-dependent activation of soluble heme-containing guanylate cyclase (sGC) and the consequent increase in the second messenger cyclic guanosine monophosphate (cGMP) [Citation5,Citation6]. However, several investigations reveal that the mechanism(s) of action of CO could involve other potential targets [Citation7]. Among the various biological effects reported for CO, one interesting aspect is the role this gaseous mediator might play in thermoregulation [Citation8–10]. Previous studies have shown that the thermoregulatory effects of CO take place in the central nervous system (CNS) [Citation9]. Specifically, body core temperature (Tb) measured by biotelemetry in freely moving rats increased after an intracerebroventricular administration of heme-lysinate, which induces the HO-1 pathway [Citation9]. This response was attenuated by pre-treatment with a heme oxygenase inhibitor, indicating that a product of this pathway has a pyretic action in the CNS. The fact that neither biliverdin nor iron, the two other products of heme degradation by heme oxygenase, had any effect on Tb once injected into the cerebral ventricles indirectly suggested a possible role of CO in thermoregulation. Notably, central administration of CO-saturated artificial cerebral spinal fluid has been shown to cause an increase in Tb, which could be blocked by indomethacin indicating that the pyrogenic role of CO in CNS is dependent on prostaglandin production [Citation11]. However, the putative effect of systemically delivered CO on Tb regulation has not been fully investigated [Citation12].
The advent of CO-releasing molecules (CO-RMs), a class of compounds that carry and deliver controlled amounts of CO to biological systems, has greatly facilitated the investigations on CO gas and confirmed their therapeutic potential in different models of disease [Citation7,Citation13]. Interestingly, it has been reported that CORM-401, a pharmacologically active compound that releases CO with high efficiency [Citation14,Citation15], uncouples mitochondrial respiration in adipocytes and other cell types and reduces body weight gain in obese mice orally treated with this compound [Citation16,Citation17]. As mitochondrial uncoupling is well-known to be associated with dissipation of energy by heat production [Citation18,Citation19], these findings might have important implications for thermoregulatory responses mediated by CO. In the present study, we tested for the first time the effect of oral administration of CORM-401 on Tb in rats and assessed whether a thermogenic response mediated by CO is maintained by non-shivering thermogenesis measured as whole-body oxygen consumption in these animals.
Methods
Ethical approval
Experimental protocols used herein were approved by the Institutional Ethical Committee on Animal Experimentation of the Dental School of Ribeirão Preto, University of São Paulo (#2017.1.585.58.9). In total, 20 adult males Wistar rats 7- to 8-week-old (weighing 234 ± 9 g) from the Animal Care Facility of the University of São Paulo, Campus of Ribeirão Preto, Brazil, were used in our experiments. The animals had free access to regular food and water. Rats were housed in plexiglass cages and kept in a room with temperature regulated within 22–24°C and a 12 h-12 h light-dark cycle.
Measurements of blood carbon monoxy hemoglobin (COHb) levels
To verify that CORM-401 treatment effectively delivers CO into the blood circulation in rats, the levels of COHb were determined as previously described by our group [Citation20]. Briefly, blood (5 μl) collected from the rat tail vein at different time points after oral administration with CORM-401 (30 mg.kg−1) was added to a cuvette containing 4.5 ml deoxygenated tris(hydroxymethyl) aminomethane solution and spectra were recorded. The percentage of COHb was calculated based on the absorbance at 420 and 432 nm, with the reported extinction coefficients for rat blood [Citation21].
Tb recordings
Rats were deeply anesthetized with a mixture of ketamine (100 mg.kg−1) and xylazine (10 mg.kg−1) and after the absence of withdrawal reflex to a pinch of tail and paw a calibrated temperature datalogger (SubCue, Calgary, AB, Canada) was inserted into their abdominal cavity through an aseptic median laparotomy. Forty-eight hours after the surgery, Tb was recorded in freely-moving rats from 60 min before oral gavage of either vehicle (PBS) or CORM-401 (30 mg.kg−1) at 10 min intervals by a programmed data logger and extracted using SubCue software.
Tail skin temperature and HLI measurements
To measure the cutaneous temperature (Tsk) of the middle third of the tail length we used an infrared thermographic sensitivity camera (FLIR ONE; FLIR Systems Inc, Wilsonville, OR, USA) at 60 and 180 min after oral gavage administration of either vehicle or CORM-401. Ambient temperature (Ta) was maintained at 28.6 ± 0.4°C. To calculate heat loss index measurements (HLI) the infrared photos, Tb recording and Ta were acquired simultaneously using the formula: HLI = (Tsk − Ta) (Tb − Ta)−1 [Citation22,Citation23]. To access the thermo-effector mechanisms the HLI ranged from 0 (maximum vasoconstriction) to 1 (maximum vasodilatation).
Oxygen consumption (VO2), carbon dioxide production (VCO2) and respiratory exchange ratio (RER)
Metabolic and non‐shivering thermogenesis studies were performed placing each animal in an individual sealed chamber (6 L). Rats were placed in this individual unit for a 30 minutes’ acclimation period. The air samples passed through a gas analyzer (Gas analyzer ML206, ADInstruments, NSW, Australia). Consumed O2 (VO2) and produced CO2 (VCO2) were collected at 60 and 180 min after oral gavage administration of Vehicle or CORM-401 and measurements were utilized to calculate the respiratory exchange ratio (RER) by dividing VCO2 by VO2. Measures were adjusted by body weight and analyzed as mL/kg/min.
Femoral artery catheterization and cardiovascular recordings
Rats had an arterial catheter implanted in the femoral artery with implantation of a polyethylene catheter (PE-10 connected to PE-50 tubing; Clay Adams, Parsippany, NJ, USA, Intramedic, Becton Dickinson, Sparks, MD, USA) filled with saline in the descending aorta (for hemodynamic recordings), and then were pulled up through a subcutaneous track through the nape of the neck and then surgical incisions were sutured. Animals recovered and acclimated individually for 48 hours in the recording room before blood pressure recordings in unanesthetized unrestrained freely behaving rats. To avoid clogging of the catheters, they were flushed with heparinized saline (0.2 mL) one day before the recordings and on the day of the experiments before connecting the rats to the pressure transductor [Citation24].
Statistics
Statistical analysis was performed using Prism (Version 7.03, La Jolla, CA, USA). To compare the differences between groups, we used two-way ANOVA with the Bonferroni’s post hoc tests or unpaired t test as required. Data are represented as mean ± SEM. Statistical significance was considered at a level of p < 0.05.
Results
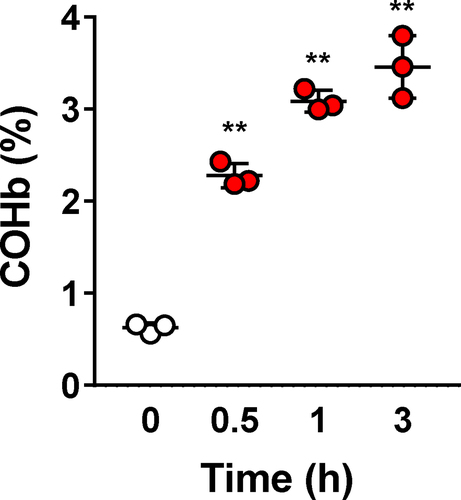
Oral administration of CORM-401 in rats resulted in a significant and time-dependent increase in COHb levels indicating that CO is delivered systemically to the animals and confirming previous studies conducted in mice [Citation17] (P = 0.0013, ). Tb was recorded in conscious, freely moving rats combined with the evaluation of the eventual thermo-effector mechanisms (non‐shivering thermogenesis and heat loss index) involved in the thermoregulatory responses to oral CORM-401.
Figure 1. Time course of blood carbon monoxy hemoglobin (COHb) levels after CORM-401 treatment. Rats (n = 3) were administered with 30 mg.kg−1 and blood collected at different time points to measure COHb levels by a spectrophotometric method. **p < 0.01 vs. time 0.
Oral CORM-401 administration caused a significant increase in Tb when compared with time 0 and with rats that received vehicle (Vehicle: −0.35 ± 0.13 Δ°C vs. CORM-401: 0.40 ± 0.20 Δ°C P = 0.0227, F1, 12 = 6,821, ). The significant increase in Tb in response to CORM-401 was also marked by an enhanced thermal index (TI) that was significantly higher in rats that received CORM-401 (45 ± 14°C min−1) compared to vehicle rats (−26 ± 8°C min−1, P = 0.0007, ).
Figure 2. Time course of changes in deep body temperature (Δ Tb in °C) in response to vehicle or CORM-401 (panel a). Thermographic images of representative rats that received vehicle or CORM-401 at 1 h and 3 h (panel b). Arrows indicate the site of tail skin temperature assessment. Average and individual values of thermal indexes (TI, °C x min) from 60 to 180 min after vehicle or CORM-401 (panel c). Heat loss index of rats that received vehicle or CORM-401 (panel d). Oxygen consumption (VO2) of rats that received vehicle or CORM-401 (panel E). (n = 7–10 vehicle and n = 5–7 CORM-401 treated animals). **p < 0.01, ***p < 0,001, ****p < 0.0001.
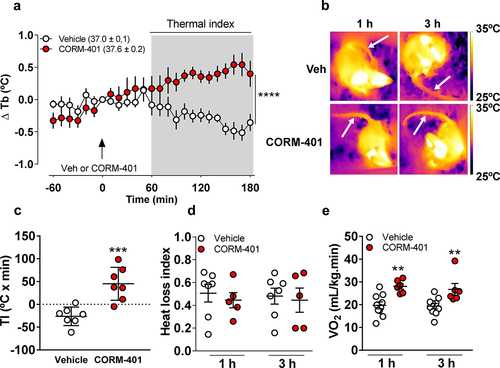
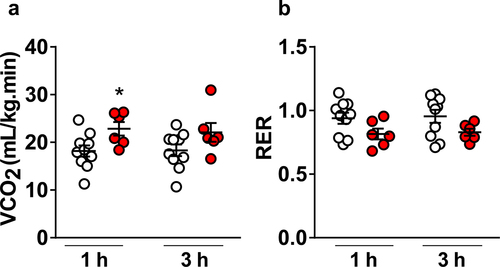
To further characterize the thermoregulatory responses stimulated by CORM-401, we collected thermal images of each experimental group are presented in . Rats that received CORM-401 showed no differences in HLI in comparison with vehicle (1 h – Vehicle: 0.51 ± 0.08 vs. CORM-401: 0.45 ± 0.07; 3 h – Vehicle: 0.48 ± 0.07 vs. CORM-401: 0.45 ± 0.11, P = 0.8219, ). VO2 was measured as an index of non-shivering thermogenesis and metabolism. Consistently with a rise in Tb, VO2 was significantly higher in rats that received oral CORM-401 in comparison with vehicle (1 h – Vehicle: 20 ± 1 mL kg−1 min−1, vs. CORM-401: 28 ± 1 mL kg−1 min−1, P = 0.0025; 3 h – Vehicle: 19 ± 1 vs. CORM-401: 27 ± 2, P = 0.0072, ). These findings are consistent with the notion that CORM-401 leads to elevation of Tb by increasing non-shivering thermogenesis as a major thermoeffector mechanism. Besides VO2, CO2 production was also monitored simultaneously and in the very same metabolic cages. CORM-401 significantly increased VCO2 at 1 h (Vehicle: 18 ± 1 mL kg−1 min−1, vs. CORM-401: 23 ± 1 mL kg−1 min−1, P = 0.0222, F1, 14 = 6,607) and this increase was not observed at 3 h after CORM-401 administration (Vehicle: 18 ± 1 vs. CORM-401: 22 ± 2, P = 0.1446, ). On the other hand, RER was similar between rats that received Vehicle or CORM-401 (1 h – Vehicle: 0.94 ± 0.04, vs. CORM-401: 0.82 ± 0,04, P = 0.1531; 3 h – Vehicle: 0.96 ± 0.05 vs. CORM-401: 0.83 ± 0.03, P = 0.1462, ).
Figure 3. Average and individual values of carbon dioxide production (VCO2) (panel A) and respiratory exchange ratio (RER) of rats that received vehicle or CORM-401 (panel B) (n = 10 vehicle and n = 6 CORM-401 treated animals). *p < 0.05.
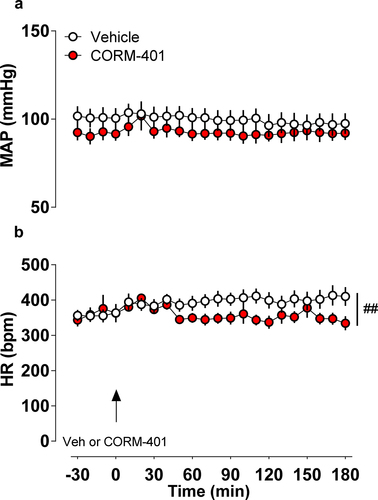
Finally, to verify if cardiovascular parameters are affected by CORM-401, blood pressure and heart rate recordings were performed simultaneously to Tb measurements. The data showed that the levels of MAP (Vehicle: 98 ± 6 mmHg, vs. CORM-401: 92 ± 4 mmHg, P = 0.3733, ) were similar in all groups evaluated. On the other hand, there was a significant interaction between drug and time in HR (Vehicle: 410 ± 26 bpm, vs. CORM-401: 334 ± 20 bpm at 180 minutes after treatment, P = 0.0065, F21, 231 = 2,017, ).
Figure 4. Time course of mean arterial pressure (MAP in mmHg, panel a) and heart rate (HR in bpm, panel b) were continuously monitored throughout 180 min in rats (n = 8 vehicle and n = 5 CORM-401 treated animals) in response to vehicle or CORM-401 administration. ## p < 0.01 interaction between treatment and time.
Discussion
In this study, we have shown that oral administration of CORM-401 to rats leads to an increase in Tb caused by augmented non-shivering thermogenesis without affecting neither the amount of heat loss through the tail skin nor blood pressure. Thus, the present data are consistent with the notion that delivery of controlled amounts of CO to the body causes an increase in Tb associated with an elevated heat production, indicating that this thermoeffector is specifically activated after systemic CO liberation. Moreover, these thermoregulatory responses were not associated with any significant changes in arterial blood pressure. Non-shivering thermogenesis is defined as an increased metabolic heat production (above the basal metabolism) that is not associated with muscle activity but rather resulting mainly from the increased metabolism of brown fat [Citation25].
Besides producing energy (ATP), mitochondria also generate heat which is crucial for thermoregulation, metabolism and body weight control. Thus, it is not surprising that mitochondrial thermogenesis has become an important pharmacological target in the development of tools, such as CORM-401, to treat metabolic disorders [Citation17]. This specialized thermogenic pathway relies on a controlled proton (H+) leak across the inner mitochondrial membrane, which dissipates energy from the electrochemical H+ gradient. Even though the mechanisms of H+ leak across the inner mitochondrial membrane are far from fully understood, heat is thought to be generated by uncoupling proteins (UCPs) that form hydrophilic and selective pores through the membrane letting H+ down its gradient, without generating ATP, but allowing energy to be dissipated as heat. This uncoupling H+ current is physiologically regulated by free fatty acids that activate UCPs allowing H+ leak [Citation26], and recent evidence indicates that CO is also able to modulate this pathway [Citation17]. In fact, a transient uncoupling activity of CO associated with a switch in adipose tissue metabolism from oxidative phosphorylation to glycolysis of following oral administration of CORM-401 was recently shown to reduce body weight gain and increase insulin sensitivity as well as glucose tolerance in obese mice fed a high-fat diet [Citation17].
One may not exclude the possibility of an interaction between peripheral CO and sympathetic nerve fibers and/or beta-3 adrenergic receptors that are known to be crucial for activation of non-shivering thermogenesis in brow adipose tissue after noradrenaline is released [Citation27]. This hypothesis has never been tested in the rat’s brown adipose tissue, but cerebral arterioles from newborn pigs seem to be affected by an endothelium-dependent dilation that involves stimulation of CO production via the HO pathway in the endothelium [Citation28].
As mentioned formerly, mechanisms involved in the role of CO in the Tb control were investigated in previous studies [Citation29,Citation30], but the mechanistic differences between central and systemic delivery of controlled amounts of CO were not addressed before. In discussing CO-related increase in Tb, our previous publications documented that CO resulting from heme metabolism by heme oxygenase in the CNS plays a significant role in fever generation, but not in body temperature regulation under euthermia and that this thermogenic effect of CO is independent of prostaglandin [Citation8,Citation10]. However, the role of peripheral CO in thermoregulatory responses was not assessed in those reports. In the present study, we showed that peripheral CO acts increasing body temperature due to non-shivering thermogenesis. Whether CO liberated in the systemic circulation is crossing the blood-brain barrier activating the CNS thermoregulatory areas such as the anteroventral region of the hypothalamus or if this effect on non-shivering thermogenesis vasculature is local are important matters deserving further investigation.
CO is endogenously produced in the body and acts through interconnected mechanisms inducing vasodilation in the brain and regulating systemic blood pressure [Citation31,Citation32]. In the CNS, CO interacts with several nuclei of the brain, including the nucleus tractus solitarius affecting blood pressure regulation [Citation33]. In the present study, we observed that CO-induced thermoregulatory response was not associated with the emergence of tail artery vasodilatation, nor significant changes in blood pressure and a significant decrease in heart rate. Interestingly, we observed that CORM-401 administration increased oxygen consumption, heat production and CO2 production without affecting other blood pressure. The increase in metabolic activity after CORM-401 administration is consistent with previous data showing increases in CO2 production after treatment with another CO-releasing compound (CORM-A1) [Citation34]. The increase in CO-induced CO2 production could be attributed, at least in part, to elevated motor activity. Although the present experiments cannot explain why CO-induced thermoregulatory responses were not associated with changes in blood pressure, we speculate that the chosen dose of CORM-401 and route of administration lead to specific metabolic responses without affecting the activity of resistance vessels, but causing a reduction in heart rate.
The selection of the dose of CORM-401 was based on our previous published article [Citation17]. shows that the levels of COHb in rats increased from 0.6% (physiological concentrations) to 3.5% after oral administration of 30 mg/kg CORM-401. These values are very similar to the ones obtained in mice [Citation17], indicating that there is a marked increase in COHb in the blood after administration of 30 mg/kg CORM-401. We need to point out that these therapeutic levels of COHb are below the threshold of 10% obtained following CO gas inhalation in humans, which has been shown to cause no adverse effects in a clinical trial approved by the FDA [Citation35].
In summary, the present results indicate that oral CORM-401 administration leads to elevation of Tb caused by increased oxygen consumption without affecting blood pressure. In view of the recent findings that CO can uncouple mitochondrial respiration, these data may have implications for additional pre-clinical studies aiming to investigate the eventual specificity of CO signaling for therapeutic applications of CO and CO-RMs in the treatment of obesity and obesity-related disorders.
List of abbreviations
CO: carbon monoxide
Tb: body core temperature
CORM-401: CO-releasing molecule-401
HO: heme oxygenases
sGC: guanylate cyclase
cGMP: cyclic guanosine monophosphate
CNS: central nervous system
COHb: carbon monoxy hemoglobin
Tsk: cutaneous temperature
Ta: ambient temperature
HLI: heat loss index measurements
VO2: oxygen consumption
VCO2: carbon dioxide production
UCPs: uncoupling proteins
RER: respiratory exchange ratio
TI: thermal index
Author contributions
MRA, RF, RM and LGSB conceived the idea and designed the study. MRA, DB and RM performed experiments and collected data. MRA, RF, RM and LGSB analyzed the data and wrote the manuscript.
Acknowledgments
We are grateful to Mauro F. Silva for his excellent technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci Off J Soc Neurosci. 1994;14:5147–5159.
- Motterlini R. Carbon monoxide-releasing molecules (CO-RMs): vasodilatory, anti-ischaemic and anti-inflammatory activities. Biochem Soc Trans. 2007;35:1142–1146.
- Foresti R, Bani-Hani MG, Motterlini R. Use of carbon monoxide as a therapeutic agent: promises and challenges. Intensive Care Med. 2008;34:649–658.
- Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743.
- Verma A, Hirsch DJ, Glatt CE, et al. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384.
- Morita T, Perrella MA, Lee ME, et al. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci U S A. 1995;92:1475–1479.
- Motterlini R, Foresti R. Biological signaling by carbon monoxide and carbon monoxide-releasing molecules. Am J Physiol Cell Physiol. 2017;312:C302–C313.
- Steiner AA, Branco LG. Central CO-heme oxygenase pathway raises body temperature by a prostaglandin-independent way. J Appl Physiol Bethesda Md 1985. 2000;88:1607–1613.
- Steiner AA, Branco LG. Carbon monoxide is the heme oxygenase product with a pyretic action: evidence for a cGMP signaling pathway. Am J Physiol Regul Integr Comp Physiol. 2001;280:R448–457.
- Steiner AA, Colombari E, Branco LG. Carbon monoxide as a novel mediator of the febrile response in the central nervous system. Am J Physiol. 1999;277:R499–507.
- Jang C-G, Lee S-J, Yang S-I, et al. Carbon monoxide as a novel central pyrogenic mediator. Arch Pharm Res. 2002;25:343–348.
- Branco LGS, Soriano RN, Steiner AA. Gaseous mediators in temperature regulation. Compr Physiol. 2014;4:1301–1338.
- Motterlini R, Mann BE, Foresti R. Therapeutic applications of carbon monoxide-releasing molecules. Expert Opin Investig Drugs. 2005;14:1305–1318.
- Crook SH, Mann BE, Ajhm M, et al. [Mn(CO)4{S2CNMe(CH2CO2H)}], a new water-soluble CO-releasing molecule. Dalton Trans Camb Engl 2003. 2011;40:4230–4235.
- Fayad-Kobeissi S, Ratovonantenaina J, Dabiré H, et al. Vascular and angiogenic activities of CORM-401, an oxidant-sensitive CO-releasing molecule. Biochem Pharmacol. 2016;102:64–77.
- Kaczara P, Motterlini R, Rosen GM, et al. Carbon monoxide released by CORM-401 uncouples mitochondrial respiration and inhibits glycolysis in endothelial cells: a role for mitoBKCa channels. Biochim Biophys Acta. 2015;1847:1297–1309.
- Braud L, Pini M, Muchova L, et al. Carbon monoxide-induced metabolic switch in adipocytes improves insulin resistance in obese mice. JCI Insight. 2018;3:123485.
- Brand MD. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol. 2000;35:811–820.
- Divakaruni AS, Brand MD. The regulation and physiology of mitochondrial proton leak. Physiol Bethesda Md. 2011;26:192–205.
- Nikam A, Ollivier A, Rivard M, et al. Diverse Nrf2 activators coordinated to cobalt carbonyls induce heme oxygenase-1 and release carbon monoxide in Vitro and in Vivo. J Med Chem. 2016;59:756–762.
- Rodkey FL, Hill TA, Pitts LL, et al. Spectrophotometric measurement of carboxyhemoglobin and methemoglobin in blood. Clin Chem. 1979;25:1388–1393.
- Romanovsky AA, Ivanov AI, Shimansky YP. Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol Bethesda Md 1985. 2002;92:2667–2679.
- Santos BM, Francescato HDC, Turcato FC, et al. Increased hypothalamic hydrogen sulphide contributes to endotoxin tolerance by down-modulating PGE2 production. Acta Physiol Oxf Eng. 2020;228:e13373.
- Amorim MR, Moreira DA, Santos BM, et al. Increased lipopolysaccharide-induced hypothermia in neurogenic hypertension is caused by reduced hypothalamicproduction and increased heat loss. J Physiol. 2020. DOI:10.1113/JP280321
- Banet M, Hensel H, Liebermann H. The central control of shivering and non-shivering thermogenesis in the rat. J Physiol. 1978;283:569–584.
- Azzu V, Brand MD. The on-off switches of the mitochondrial uncoupling proteins. Trends Biochem Sci. 2010;35:298–307.
- Edwards MM, Nguyen HK, Dodson AD, et al. Effects of combined oxytocin and Beta-3 receptor agonist (CL 316243) treatment on body weight and adiposity in male diet-induced obese rats. Front Physiol. 2021;12:725912.
- Fiumana E, Parfenova H, Jaggar JH, et al. Carbon monoxide mediates vasodilator effects of glutamate in isolated pressurized cerebral arterioles of newborn pigs. Am J Physiol Heart Circ Physiol. 2003;284:H1073–1079.
- Collins MG, Hunter WS, Blatteis CM. Factors producing elevated core temperature in spontaneously hypertensive rats. J Appl Physiol Bethesda Md. 1987;1985(63):740–745.
- Berkey DL, Meeuwsen KW, Barney CC. Measurements of core temperature in spontaneously hypertensive rats by radiotelemetry. Am J Physiol. 1990;258:R743–749.
- Leffler CW, Parfenova H, Jaggar JH. Carbon monoxide as an endogenous vascular modulator. Am J Physiol - Heart Circ Physiol. 2011;301:H1–H11.
- Motterlini R, Gonzales A, Foresti R, et al. Heme oxygenase-1-derived carbon monoxide contributes to the suppression of acute hypertensive responses in vivo. Circ Res. 1998;83:568–577.
- Stec DE, Drummond HA, Vera T. Role of carbon monoxide in blood pressure regulation. Hypertension. 2008;51:597–604.
- Hosick PA, AlAmodi AA, Storm MV, et al. Chronic carbon monoxide treatment attenuates development of obesity and remodels adipocytes in mice fed a high-fat diet. Int J Obes 2005. 2014;38:132–139.
- Fredenburgh LE, Perrella MA, Barragan-Bradford D, et al. A phase I trial of low-dose inhaled carbon monoxide in sepsis-induced ARDS. JCI Insight. 2018;3:124039.
