ABSTRACT
Meal temperature is known to affect gastric emptying and appetite. While protein intake is recommended for older age, gastric emptying is delayed with age, resulting in loss of appetite. This study aimed to investigate whether adjusting the temperature of protein-containing drinks could improve gastric emptying and appetite in older individuals. Twenty male and female participants aged 65 years and older underwent three one-day trials in random order. Participants visited the laboratory after a 10-hour fast and consumed 200 mL of protein-containing drink dissolved in 13C-sodium acetate at 4°C, 37°C, or 60°C in a 3-minute period. Then, participants sat in a chair for 90 minutes to measure gastric emptying rate by the 13C-sodium acetate breath test and subjective appetite by a visual analog scale. The results showed that 37°C and 60°C drinks had faster gastric emptying at 5 and 10 min after ingestion than did the 4°C drink (trial-time interaction, p = 0.014). Tmax-calc, an indicator of gastric emptying rate, tended to be faster for the 37°C and 60°C drinks than for the 4°C drink (49.7 ± 17.5 min vs. 44.1 ± 18.5 min vs. 45.3 ± 25.8 min for the 4°C, 37°C, and 60°C, respectively; p = 0.085). There were no significant differences in the change in hunger from baseline among the three different temperature drinks (p > 0.05). Only in the 60°C trial, a shorter gastric emptying time was associated with greater hunger (r=-0.554, p = 0.021). These findings suggest that hot protein-containing drinks may accelerate gastric emptying and contribute to rapid nutrient intake and increased appetite in older adults.
Introduction
Meal temperature is known to affect digestive function and appetite [Citation1–9]. Thermoreception of hot or cold drinks has been suggested to occur at thermoreceptors in the abdominal cavity rather than in the oral cavity [Citation10,Citation11]. Recent studies have shown that meal temperature alters the rate of gastric emptying, indicative of gastric digestion [Citation1,Citation6,Citation8]. Although it has been thought that drinks colder or hotter than body temperature inhibit gastric emptying [Citation8,Citation9], recent studies have reported that the rate of gastric emptying is faster when drinking hot drinks (55–60°C) than when drinking at 4°C or 37°C [Citation1,Citation6]. Gastric emptying occurs by peristalsis of the stomach from the proximal region to the pylorus [Citation12]. This gastric motility and gastric emptying are affected by the temperature of the hot or cold meal [Citation1–9].
Promoting gastric emptying has benefits for people, such as the older population, whose digestive function is impaired [Citation13]. It has been reported that digestive function declines in older individuals, slowing the rate at which food is expelled from the stomach [Citation14]. Delayed gastric emptying can cause gastrointestinal symptoms such as delayed nutrient absorption and stomach bloating, which can be a concern in the nutritional intake of the older population. Increased protein intake is associated with the maintenance or increase in lean body mass [Citation15] and has a protective role in preventing frailty in older populations [Citation16]. The consumption of protein-containing drinks has been suggested as a potential intervention to address this issue [Citation17]. However, previous studies have reported slower gastric emptying rates after consumption of protein-containing drinks compared to other nutritional drinks [Citation18]. To approach this issue, applying the knowledge that gastric emptying is enhanced by hot drinks, hot protein-containing drinks may be beneficial to promote gastric emptying in the older population.
Additionally, drink temperature is known to affect appetite via changes in gastric motility [Citation2,Citation4]. Prior research has shown that the consumption of a hot drink increases gastric contractions and subsequent energy intake from a meal [Citation2,Citation4]. As aging decreases appetite, there is a risk of malnutrition, which results in decreased energy intake from the diet [Citation19]. Therefore, the consumption of hot protein drinks may contribute to the prevention of malnutrition by stimulating the appetite of the older population.
The aim of this study is to investigate the effect of the temperature of protein-containing drinks on gastric emptying rate and appetite in older adults aged 65 years and older. The hypothesis is that the consumption of a protein-containing drink at 60°C will accelerate gastric emptying and increase hunger compared to the consumption of a drink at 4°C and 37°C.
Materials and methods
The Ethics Committee of the Tokyo Institute of Technology, Japan, approved the present study (approval number: 2022248). This study was registered in the UMIN Clinical Trial Registry (UMIN: 000049751) and conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Written informed consent for participation in the study was obtained from 20 participants (male-to-female ratio = 10:10) aged 65 years and older. The physical characteristics of the participants were as follows: age: 72.1 ± 3.9 years; height: 1.60 ± 0.09 m; body mass: 57.8 ± 10.1 kg; and body mass index: 22.3 ± 2.3 kg/m2. The exclusion criteria were as follows: individuals with diabetes or renal disorders that required dietary restrictions, with gastrointestinal diseases that affect food digestion, with difficulty taking protein drinks orally, or those taking antipsychotic or hormonal medications that may affect their appetite.
The participants underwent three one-day laboratory-based trials in a randomized order. From the day before the first trial, the participants weighed and recorded all dietary intakes and subsequently replicated these dietary intakes for dinner the day before the second and third trials. We analyzed food diaries using a software program (Excel Eiyou-kun, ver 9.0; Kenpakusha, Tokyo, Japan) to determine the energy intake and macronutrient content. All the participants were asked to maintain their normal eating habits during the trials. After at least 10 h of overnight fasting, participants arrived at the laboratory at 9:00 a.m. On arrival at the laboratory, the participants were briefed about each trial in a chair in a laboratory room. The room temperature and humidity were maintained at 22.1 ± 1.1°C and 41.0 ± 3.3%, respectively.
To estimate digestion and absorption rates, we collected breath samples before drink ingestion using a large-volume bag (PAYLORI-BAG 1.3 L; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan). The participants completed a visual analog scale (VAS) [Citation20] to assess their appetite and feelings of stomach discomfort before ingesting the drink. Participants ingested 200 mL of a nutrient drink containing 10.8 g carbohydrate and 15 g milk protein (ZAVAS protein drink; Meiji, Tokyo, Japan) during 3 min at three different temperatures (4°C, 37°C, and 60°C), 30 min after arrival at the laboratory. To maintain the set temperature, protein-containing drinks were served in thermal tumblers. The temperature of the protein-containing drinks was measured using an electric thermometer (TT-508N; TANITA Corp., Tokyo, Japan). The temperature of the drinks was set with reference to a previous study [Citation6]. 4°C was employed as the cold temperature, 37°C as similar to the intragastric temperature [Citation21], and 60°C as the warm temperature. Participants swallowed the drinks promptly without oral retention. These drinks contained 100 mg of dissolved 13C-sodium acetate, and the interval between trials was at least 6 days. The composition of the 200 mL protein-containing drink was 0.43 MJ, 58.8% protein, 0.0% fat, and 41.2% carbohydrate. After ingesting the protein-containing drink, the participants were placed in a sitting position for 1.5 h. To measure the digestion and absorption rates, we collected exhaled breath samples using small-volume bags (PAYLORI-BAG 20; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) at 5- and 10- min intervals. The participants completed the VAS every 30 min after drink ingestion to assess their perceptions of appetite and feelings of stomach discomfort.
The gastric emptying rate of the protein-containing drinks was assessed using the 13C-sodium acetate breath test [Citation22]. The mass of 13C-sodium acetate was measured using an electronic scale (AXZ1204; AS ONE, Osaka, Japan). 13C-sodium acetate breath testing strongly correlates with the data obtained through scintigraphy, which is the gold standard for measuring the gastric emptying rate [Citation22–24]. Exhaled breath samples were measured at baseline and at 5- and 10-min intervals after the ingestion of protein-containing drinks. The 13CO2 ratio was measured using an isotope ratio mass spectrometer (POCone Plus; Otsuka Electronics Co., Ltd., Tokyo, Japan). CO2 production was assumed to be 300 mmol/m [Citation2] body surface area per hour [Citation22], and the body surface area was calculated from body mass and height. To evaluate gastric emptying, we used %dose/h and Tmax-calc. To evaluate changes in gastric emptying over time, 13C excretion rate (%dose/h) was used by based on the percentage of 13CO2 excreted per unit time from the Δ [Citation13]CO2/ [Citation12]CO2 value in exhaled breath, considering the CO2 production rate. To compare gastric emptying rates between trials at once, the time of maximum 13C excretion rate (Tmax-calc) was determined by referring to the lag phase of the previous studies [Citation6,Citation22]. Participants completed the VAS before and every 30 min after drink ingestion to assess their perceptions of appetite (i.e. hunger, fullness, desire to eat, and sweets) and feelings of stomach discomfort (i.e. “does your stomach feel uncomfortable?” and “do you feel gastric distension?’). Hunger and the desire to eat are different concepts; hunger represents a more physiological desire, and the desire to eat can occur without hunger [Citation25]. The verbal anchors “not at all” and “extremely” were placed 0 and 100 mm on the VAS, respectively.
The sample size was estimated using G*Power 3.1 [Citation26] using data from a previous study that investigated the effects of protein-containing drink temperatures on gastric emptying [Citation1]. To detect changes in gastric emptying with a power of 80% and an alpha level of 5%, we needed a sample size of ≥ 18 participants. We assumed a dropout rate of 10%, and set a sample size of 20. Data were analyzed using Predictive Analytics Software for Windows (IBM SPSS Statistics 23.0, SPSS Japan, Inc., Tokyo, Japan). The Shapiro – Wilk test was used to check for the normality of distribution. The normally distributed 13C emission rate (%dose/h), appetite scores, and feelings of stomach discomfort were analyzed using a trial × time repeated measures two-way analysis of variance (two-way-ANOVA). Tmax-calc, which was non-parametric, was analyzed using the Friedman test. When significant main or interaction effects were detected, the Bonferroni test was performed. As in the previous study [Citation27], there were no significant differences in gastric emptying by sex among older males and females in this study, thus, a combined male-female sample was used for all analyses. The correlation coefficient was determined using Pearson’s product-moment tests between Tmax-calc and the area under the curve of hunger. Data are expressed as means ± standard deviation (SD). Statistical significance was set at p < 0.05. A p-value of less than 0.1 was set by the authors as the threshold for a trend in this study.
Results
The mean energy intake for dinner on the day before the experiment was 2.3 ± 0.6 MJ. The energy intake equated to 31.8 ± 13.8% (20.1 ± 12.0 g) from fat, 48.7 ± 16.9% (67.1 ± 32.6 g) from carbohydrates and 23.8 ± 10.0% (32.0 ± 13.3 g) from protein.
shows body mass, appetite scores, and feelings of stomach discomfort at baseline. There were no significant differences in fullness, Desire for sweets, desire to eat, stomach discomfort, and gastric distension between the 4°C, 37°C, and 60°C trials at baseline. Repeated measures one-way analysis of variance showed that p-values for body mass and hunger were p < 0.1 between trials. Meanwhile, the post-hoc test showed no significant differences between trials.
Table 1. The body mass, appetite score and feelings of stomach discomfort at baseline.
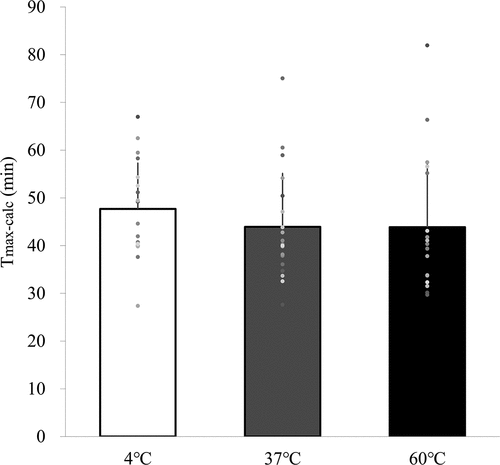
The % dose/h values for the 4°C, 37°C, and 60°C trials from the start of the trial to 90 min after drink ingestion are shown in . %dose/h and Tmax-calc were adopted as an assessment of gastric emptying based on the standard method of measuring gastric emptying for 90 minutes. There was a significant interactive effect of trial and time on the %dose/h value (p = 0.014). At 5 min after consumption, there was faster gastric emptying of a drink at 37°C (p = 0.008) and 60°C (p = 0.007) than of a drink at 4°C for 5 min (2.5 ± 0.4, 4.8 ± 0.5, and 4.9 ± 0.6 for the 4°C, 37°C, and 60°C trials, respectively). At 10 min after consumption, there was faster gastric emptying of a drink at 37°C (p = 0.019) and 60°C (p = 0.042) compared to 4°C (7.8 ± 1.1 vs. 11.6 ± 1.1 vs. 12.0 ± 1.3 for the 4°C, 37°C, and 60°C trials, respectively; p < 0.05). The Tmax-calc for the 4°C, 37°C, and 60°C trials is shown in . Tmax-calc was significantly different between trials (p = 0.041). A post-hoc test showed that gastric emptying time tended to be faster at 37°C and 60°C than at 4°C (p = 0.085). The values of Tmax-calc at 4, 37, and 60°C were 49.7 ± 17.5 min, 44.1 ± 18.5 min and 45.3 ± 25.8 min, respectively.
Figure 1. The % dose/h values for the 4°C, 37°C, and 60°C trials from the start of the trial to 90 min after drink ingestion. N = 20, data are means ± SD. Means were compared using two-factor ANOVA. 4°C: protein-containing drink intake at 4°C, 37°C: protein-containing drink intake at 37°C, 60°C: protein-containing drink intake at 60°C. There was a significant interactive effect of trial and time on the %dose/h value at 5 min (p < 0.05) and 10 min after ingestion (p < 0.05). * significantly different from the 4°C, p < 0.05.
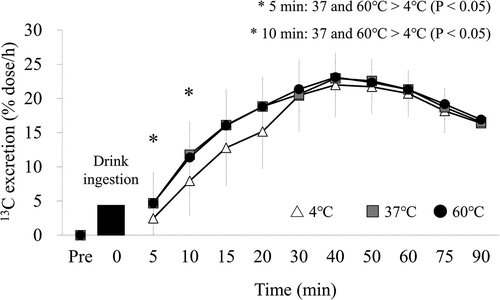
Figure 2. The Tmax-calc for the 4°C, 37°C, and 60°C trials. N = 20, data are means ± SD. 4°C: protein-containing drink intake at 4°C, 37°C: protein-containing drink intake at 37°C, 60°C: protein-containing drink intake at 60°C. Means were compared using Friedman test followed by a Bonferroni multiple-comparison test.
shows the changes from baseline (Δ) for appetite scores and gastric discomfort. There were no significant differences between trials for hunger, fullness, desire to eat, desire for sweets, stomach discomfort, and stomach distension (p > 0.05).
Table 2. Changes from baseline (Δ value) in subjective appetite scores and gastric discomfort.
There was no significant relationship between gastric emptying rate and hunger in the 4°C (r = −0.114, p = 0.643) and 37°C trials (r = −0.158, p = 0.518). Only for the 60°C trial, there was a negative relationship between the time taken for gastric emptying and hunger at 90 minutes after consumption of drinks. Thus, shorter gastric emptying times were associated with higher hunger (r = −0.554, p = 0.021).
Discussion
This study revealed that ingesting protein-containing drinks at 60°C and 37°C resulted in a faster gastric emptying rate than ingesting at 4°C in participants aged 65 and above. The %dose/h results indicate that the difference in gastric emptying due to drink temperature appears as soon as 5–10 min after drink ingestion. Only for the 60°C trial, shorter gastric emptying times were associated with higher hunger. To our knowledge, this is the first study to indicate that drink temperature plays a role in regulating digestive speed in older people who may have reduced digestive function and that rapid gastric emptying in 60°C drinks may increase hunger. The findings of this study are clinically important for incorporating temperature factors into dietary strategies for older people who may have reduced digestive function.
Three previous studies investigated the impact of cold (<10°C), body temperature, and hot (>50°C) drinks on gastric emptying rate in young individuals [Citation6,Citation8,Citation9], with no consistent results on the effect of temperature on gastric emptying rate in young individuals. Previous study found that hot drinks (60°C) led to a higher gastric emptying rate than drinks at body temperature [Citation6]. In contrast, cold drinks result in a slower gastric emptying rate than hot or body temperature drinks [Citation8]. The present study showed different results from the former study on gastric emptying in the older adults in relation to hot and body temperature drinks, as no significant difference was observed in the rate of gastric emptying, while cold drinks exhibited delayed gastric emptying compared to hot and body temperature drinks.
The age of the participants may explain the different results for the gastric emptying rate. Ingested food is temporarily stored in the stomach and subjected to peristaltic contractions of the stomach muscles, which mix the food with gastric juice before its expulsion into the small intestine. Studies suggest that the rate of gastric evacuation to the small intestine decreases with age [Citation14,Citation28], leading to an increase in food retention in the proximal portion of the stomach [Citation29]. This decrease in gastric motility with age may play a role in the relationship between drinking temperature and gastric emptying rate.
Age-related changes in thermoreceptors that sense the drink temperature may also explain the differences in the rate of gastric emptying. Gastric emptying is regulated by thermoreceptors in the stomach, which detect changes in the intragastric temperature [Citation30]. TRPV2, which is activated by temperatures above 52°C, promotes gastric emptying by stimulating gastric contraction [Citation31]. Conversely, as shown in studies on TRPM8 receptor activation and gastric contraction, cooling the stomach of rats increased the number of gastric fundus contractions up to 15°C of cooling, while cooling of more than 15°C decreased the number of gastric fundus contractions [Citation32]. TRPA1, activated at 17°C, delays gastric emptying via serotonergic pathways [Citation33]. While these studies were conducted in rodents and are limited in their translation to the human gastrointestinal tract, the slower gastric emptying observed in drinks at 4°C compared to those at 37°C and 60°C may be related to changes in TRPM8 and TRPA1 activities due to gastric cooling. This thermosensitivity is affected by aging [Citation34]. Meanwhile, the effect of aging on thermoreceptors in the gastric mucosa is unknown. Studies have shown a decrease in thermosensitivity due to reduced thermoreceptor density and epidermal blood flow in the skin [Citation34]. This change may be a contributing factor to the difference in drink temperature and gastric emptying rate.
Only in the 60°C trial, a faster gastric emptying rate was associated with higher hunger. Gastric emptying is controlled by gastric peristalsis, and gastric emptying may have been enhanced by active gastric contractions in the 60°C trial. Previous studies have reported that gastric contractions temporarily increase after consumption of a 60°C drink, leading to increased energy intake from the subsequent meal [Citation4]. Gastric motility has been reported to be associated with hunger [Citation35]. In the present study, hunger may have increased after consumption of the 60°C drink via increased gastric contractions.
The limitation of this study is that the chronic effects of varying drink temperature are unclear. Future research should explore the absorption of protein in the small intestine and muscle uptake following hot-drink ingestion, which accelerates gastric emptying, and conduct long-term studies to investigate its potential in mitigating sarcopenia and osteoporosis.
In conclusion, in males and females aged ≥65 years, ingestion of a hot protein-containing drink at 37°C or 60°C facilitated gastric emptying compared to ingestion of a protein-containing drink at 4°C. In the ingestion of protein-containing drinks as a strategy for protein supplementation in older age, drinking temperatures above body temperature may promote early digestion.
Acknowledgments
We would like to thank Dr. Kashima of Prefectural University of Hiroshima for his support for the measurement of gastric emptying. This research was partially supported through the Assistant Staffing Program by the Work-Life Balance Support Unit, Diversity Promotion Office, Tokyo Institute of Technology.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Fujihira K, Takahashi M, Shimamura K, et al. Effects of different temperatures of carbohydrate-protein-containing drinks on gastric emptying rate after exercise in healthy young men: randomized crossover trial. J Physiol Anthropol. 2021;41(1):37. DOI:10.1186/s40101-022-00311-2
- Fujihira K, Hamada Y, Haramura M, et al. The effects of different temperatures of post-exercise protein-containing drink on gastric motility and energy intake in healthy young men. Br J Nutr. 2022;127(5):782–790. DOI:10.1017/S0007114521001392
- Remes-Troche JM. “Too hot” or “too cold”: effects of meal temperature on gastric function. Dig Dis Sci. 2013;58(9):2439–2440. DOI:10.1007/s10620-013-2789-4
- Fujihira K, Hamada Y, Yanaoka T, et al. The effects of water temperature on gastric motility and energy intake in healthy young men. Eur J Nutr. 2020;59(1):103–109. DOI:10.1007/s00394-018-1888-6
- Baskentli S, Block L, Morrin M. The serving temperature effect: food temperature, expected satiety, and complementary food purchases. Appetite. 2021;160:105069. DOI:10.1016/j.appet.2020.105069
- Mishima Y, Amano Y, Takahashi Y, et al. Gastric emptying of liquid and solid meals at various temperatures. J Gastroenterol. 2009;44(5):412–418. DOI:10.1007/s00535-009-0022-1
- Sun WM, Penagini R, Hebbard G, et al. Effect of drink temperature on antropyloroduodenal motility and gastric electrical activity in humans. Gut. 1995;37(3):329–334. DOI:10.1136/gut.37.3.329
- Sun WM, Houghton LA, Read NW, et al. Effect of meal temperature on gastric emptying of liquids in man. Gut. 1988;29(3):302–305. DOI:10.1136/gut.29.3.302
- Troncon LE, Iazigi N. Effect of test meal temperature on the gastric emptying of liquids. Braz J Med Biol Res. 1988;21:57–60.
- Morris NB, Jay O. Staying warm in the cold with a hot drink: the role of visceral thermoreceptors. Temperature. 2017;4(2):123–125. DOI:10.1080/23328940.2017.1299667
- Morris NB, Filingeri D, Halaki M, et al. Evidence of viscerally-mediated cold-defence thermoeffector responses in man. J Physiol. 2017;595(4):1201–1212. DOI:10.1113/JP273052
- Varón AR, Zuleta J. From the physiology of gastric emptying to the understanding of gastroparesis. Rev Colomb Gastroenterol. 2010;25:219–225.
- Dumic I, Nordin T, Jecmenica M, et al. Gastrointestinal tract disorders in older age. Can J Gastroenterol Hepatol. 2019;2019:6757524. DOI:10.1155/2019/6757524
- O’Donovan D, Hausken T, Lei Y, et al. Effect of aging on transpyloric flow, gastric emptying, and intragastric distribution in healthy humans—impact on glycemia. Dig Dis Sci. 2005;50(4):671–676. DOI:10.1007/s10620-005-2555-3
- Tagawa R, Watanabe D, Ito K, et al. Dose–response relationship between protein intake and muscle mass increase: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2021;79(1):66–75. DOI:10.1093/nutrit/nuaa104
- Beasley JM, Shikany JM, Thomson CA. The role of dietary protein intake in the prevention of sarcopenia of aging. Nutr Clin Pract. 2013;28(6):684–690. DOI:10.1177/0884533613507607
- Chapman I, Oberoi A, Giezenaar C, et al. Rational use of protein supplements in the elderly—relevance of gastrointestinal mechanisms. Nutrients. 2021;13(4):1227. DOI:10.3390/nu13041227
- Giezenaar C, van der Burgh Y, Lange K, et al. Effects of substitution, and adding of carbohydrate and fat to whey-protein on energy intake, appetite, gastric emptying, glucose, insulin, ghrelin, CCK and GLP-1 in healthy older men—A randomized controlled trial. Nutrients. 2018;10(2):113. DOI:10.3390/nu10020113
- Morley JE. Decreased food intake with aging. J Gerontol A Biol Sci Med Sci. 2001;56(Supplement 2):81–88. DOI:10.1093/gerona/56.suppl_2.81
- Flint A, Raben A, Blundell JE, et al. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. DOI:10.1038/sj.ijo.0801083
- Hepburn JS, Eberhard HM, Ricketts R, et al. Temperature of the gastrointestinal tract: the effect thereon of hot and cold foods and of physical therapeutic agents. Arch Intern Med. 1933;52(4):603–615. DOI:10.1001/archinte.1933.00160040109006
- Ghoos YF, Maes BD, Geypens BJ, et al. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology. 1993;104(6):1640–1647. DOI:10.1016/0016-5085(93)90640-X
- Braden B, Adams S, Duan LP, et al. The [13C]acetate breath test accurately reflects gastric emptying of liquids in both liquid and semisolid test meals. Gastroenterology. 1995;108(4):1048–1055. DOI:10.1016/0016-5085(95)90202-3
- Chew CG, Bartholomeusz FDL, Bellon M, et al. Simultaneous 13C/14C dual isotope breath test measurement of gastric emptying of solid and liquid in normal subjects and patients: comparison with scintigraphy. Nucl Med Rev. 2003;6:29–33.
- Ciampolini M, David Lovell-Smith H, Kenealy T, et al. Hunger can be taught: hunger recognition regulates eating and improves energy balance. Int J Gen Med. 2013;6:465–478. DOI:10.2147/IJGM.S40655
- Faul F, Erdfelder E, Lang AG, et al. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. DOI:10.3758/BF03193146
- Giezenaar C, Trahair LG, Luscombe-Marsh ND, et al. Effects of randomized whey-protein loads on energy intake, appetite, gastric emptying, and plasma gut-hormone concentrations in older men and women. Am J Clin Nutr. 2017;106(3):865–877. DOI:10.3945/ajcn.117.154377
- Giezenaar C, Trahair LG, Rigda R, et al. Lesser suppression of energy intake by orally ingested whey protein in healthy older men compared with young controls. Am J Physiol - Regul Integr Comp Physiol. 2015;309(8):R845–R854. DOI:10.1152/ajpregu.00213.2015
- Rayner CK, MacIntosh CG, Chapman IM, et al. Effects of age on proximal gastric motor and sensory function. Scand J Gastroenterol. 2009;35(10):1041–1047. DOI:10.1080/003655200451153
- Taieb EO. Thermoreceptors in the digestive tract and their role. J Auton Nerv Syst. 1984;10(3–4):246–254. DOI:10.1016/0165-1838(84)90020-1
- Mihara H, Suzuki N, Yamawaki H, et al. TRPV2 ion channels expressed in inhibitory motor neurons of gastric myenteric plexus contribute to gastric adaptive relaxation and gastric emptying in mice. Am J Physiol - Gastrointest Liver Physiol. 2013;304(3):235–240. DOI:10.1152/ajpgi.00256.2012
- Mustafa S, Oriowo MA. Cooling-induced contraction of the rat gastric fundus: mediation via transient receptor potential (TRP) cation channel TRPM8 receptor and rho-kinase activation. Clin Exp Pharmacol Physiol. 2005;32(10):832–838. DOI:10.1111/j.1440-1681.2005.04273.x
- Doihara H, Nozawa K, Kawabata-Shoda E, et al. TRPA1 agonists delay gastric emptying in rats through serotonergic pathways. Naunyn Schmiedebergs Arch Pharmacol. 2009;380(4):353–357. DOI:10.1007/s00210-009-0435-7
- Guergova S, Dufour A. Thermal sensitivity in the elderly: a review. Ageing Res Rev. 2011;10(1):80–92. DOI:10.1016/j.arr.2010.04.009
- Janssen P, Berghe PV, Verschueren S, et al. Review article: the role of gastric motility in the control of food intake. Aliment Pharmacol Ther. 2011;33(8):880–894. DOI:10.1111/j.1365-2036.2011.04609.x
