ABSTRACT
Benthic aquatic insects receive the most direct impact when surface waters are perturbed. However, scarce data and understanding about activities’ effects on surface water ecosystems remain a critical challenge for water resource managers and policymakers in tropical regions. In this study, we surveyed the implications of deteriorating physical and chemical parameters on aquatic insects’ structural assemblage to ascertain the ecological health of River Hadejia in North-Western Nigerian. We sampled aquatic insects and physicochemical parameters in three stations influenced by various land-use activities such as informal settlements and agricultural activities for six months. The two-way analysis of variance (ANOVA) revealed physicochemical parameters such as transparency, depth, and nitrate were not significantly affected by sites’ land-use activities (p > .05) in the six months sampled. However, mean electrical conductivity was lowest in Station 3 (104.3 ± 8.04 µS/cm). Dissolved oxygen (DO), five days biochemical oxygen demand (BOD5) values recorded portray a relatively perturbed water system. We recorded four aquatic insects orders belonging to 11 families and taxa. Dytiscus sp. was the most abundant taxon in the study area. A total of 44, 37, and 35 individuals of aquatic insects were recorded in stations 1, 2, and 3 in the river. The Post hoc test performed for all the diversity indices were not significantly different between the studied stations (p > .05). Canonical correspondence analysis (CCA) revealed poor relationship between the physicochemical parameters and the aquatic insects. However, Gyrinus sp. was positively affected by increased water depth, showing a strong negative association with depth. Cluster analysis revealed that aquatic insects’ assemblage structures were mainly grouped by temporal factors (months) rather than spatial differences between the sites. Overall, this study provides further insights and understanding regarding land-use impacts on the ecological health of the River Hadejia, and we recommend more stringent regulations to control human pressure on the river systems within the studied area to enable surface waters in the area to sustain the provision of desired and valued ecosystem services.
Introduction
When surface waters are perturbed, benthic aquatic insect communities are among the most directly affected (Lubanga, Manyala, Siati, Yegon, & Masese, Citation2021). Their distribution, diversity, abundance, and composition are often used in assessing the ecological status of aquatic habitats (Arimoro, Odume, Uhunoma, & Edegbene, Citation2015; Edegbene, Citation2020). Among the taxa used for monitoring water bodies, the Ephemeroptera, Plecoeptera and Trichoptera (EPT) are frequently employed (Akamagwuna et al., Citation2019a; Al-Zankana, Matheson, & Harper, Citation2020). The EPT groups of aquatic insects are termed to be pollution-sensitive species whose presence portrays relatively clean, unperturbed and/or mildly disturbed waters (Abebe et al., Citation2009; Eriksen et al., Citation2021; Feio et al., Citation2021; Yung-Chul et al., Citation2016). Aquatic insects are found almost in every aquatic habitat, including lakes, streams, highly saline pools, coastal waters and estuaries, groundwater, and hot springs (Yule & Yong, Citation2004). Because of their diverse sensitivities to deteriorating water quality, they respond differently to pollution resulting from human activities (Firmiano, Castro, Linares, & Callisto, Citation2021).
Most freshwater bodies in Nigeria, River Hadejia inclusive, have been subjected to increasing human disturbance, resulting in changes in the environmental variables, consequently affecting the structural and functional ecology of the freshwater systems (Garba, Ekanem, & Garba, Citation2017; Umara, Ramlib, Arisc, Jamilb, & Tukur, Citation2019). Hence, biomonitoring method that employs aquatic insects’ structural assemblage can provide us with good insights to environmental management of freshwater ecosystems, allowing us to make quality decision toward accurate and justifiable actions regarding ecological status of freshwater ecosystems (Abowei & Sikoki, Citation2005; Asonye, Okolie, Okenwa, & Iwuanyanwu, Citation2007).
The pollution of freshwater bodies caused by domestic and industrial effluents is a common anthropogenic impact on watercourses globally, including freshwater ecosystems in Nigeria. Pollution modifies the physicochemical properties of water, affecting aquatic insects community distribution in a given water body. Lately, River Hadejia has had its fair share of anthropogenic disturbances due to incessant defecation, washing, urination, dumping of refuse, and runoff of fertilizers from nearby farming settlements, among others. This pollution problem is exacerbated by high population growth occasion by migration from neighboring communities surrounding Hadejia. Hadejia town is the headquarter of one of the five Emirates in the Jigawa State of Nigeria, with a high rate of rural-urban migration due to its commercial state. The rural-urban migrations have implications on the ecological health of the river system and its constituent’s aquatic communities. For example, previous studies in the study area have reported severe deterioration of water quality in the Hadejia River system and the surrounding wetlands, with deleterious effects on aquatic biodiversity (Ahmed, Agodzo, Adjei, Deinmodei, & Ameso, Citation2018; Umar, Ramli, Aris, Jamil, & Abdulkareem, Citation2018; Umara et al., Citation2019). The degradation of ecological health and biodiversity of the river system because of human activities have affected the sustainable delivery of desired ecosystem services for livelihood and well-being, drawing us back from achieving the global goals of clean water and sanitation for all. Rural–urban migration has been reported to be increasing exponentially in sub-Sahara Africa (Edegbene, Arimoro, & Odume, Citation2019; Parienté, Citation2017), and such migratory activities grossly affect negatively the structural and functional assemblage and diversity of aquatic biota which include plankton, macroinvertebrate and fish (Gieswein, Hering, & Lorens, Citation2019). In this study, we aimed to ascertain the implication of deteriorating physical and chemical parameters on the aquatic insect structural assemblage to determine the health of the Hadejia River. This study is pertinent due to the continued anthropogenic disturbance along the River Hadejia catchments. The study will unravel the present health status of the river, enabling river managers and other appropriate authorities to manage the river sustainably.
Materials and methods
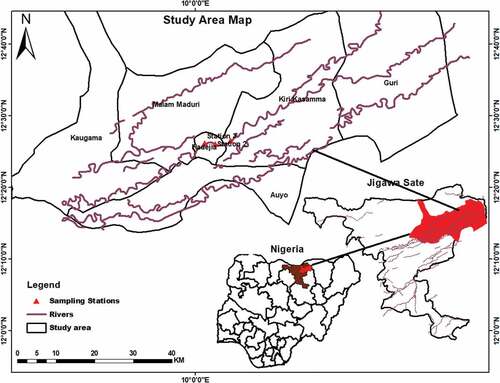
The study area
The River Hadejia is a tributary of the Yobe River situated in Jigawa State within the administrative boundaries of North-western part of Nigeria. The river and surrounding wetlands cover a catchment area of about 3500 km2 at an altitude of 152–305 m above sea level (BirdLife International, Citation2016). The River Hadejia flows through major cities including Hadejia and Nguru with various land uses that lie on or near its banks (Abubakar, Citation2009), and as such, the river is in local municipality of Hadejia and its environs. It lies between latitude 12°27 12.49’N and longitude 10°02 28.14’E in the north eastern corner of Jigawa State (). The climate in the study area is semi-arid, with average annual rainfall ranging between 600 mm to 762 mm, and the humidity is range between 25–41% (Abubakar, Citation2009; Edegbene, Citation2020). The Hadejia area temperature varies substantially between 12°C in December and January, and 40°C in March and April. Geology of the area is underlined by rock and younger sediments of the Chad formation. Vegetation in the area falls within the Sudan Savannah with extensive open grassland and a few scattered trees (Abubakar, Citation2009).
Sampling sites
We selected three study sites from the study area, based on accessibility and human activities around the area (i.e. community location). Selected sites include Station 1 located at the Aguyaka community, Hadejia Local Municipal Area (Plate 1). Human activities include washing of clothes, bathing, and subsistence fishing. Farming is the major occupation of the populace here. The biotopes are characterized by sand and loamy soil. Station 2 was located in the Yan Wanki quarters in Hadejia Local Government (Plate 2). This station is about 2 km away from station 1. Human activities include agricultural activities, washing of clothes, bathing, and fishing. The substrates here are mainly loam and clay with a sparse distribution of stones and boulders. Whereas Station 3 was located in the Bakin Gada, which is very close to the bridge that connects Hadejia to Bulangu in Kafin Hausa Local Government Area of Jigawa State (Plate 3). This station is about 1 km away from Station 2. Subsistence fishing and cattle grazing are the main land uses in this station. Other land uses include, sand dredging, bathing, defecation and washing of cars, clothes, and other households. These activities represent the leading anthropogenic disturbance that affects this site.
Physicochemical analysis
We measured temperatures using mercury in a glass thermometer, whereas transparency was determined using Secchi disc with black and white paints. Depth was measured with a calibrated rod in centimeters. Three locations where strategically marked out for the measurement of water flow velocity by timing a float (average of three trials) as it moved over distance of 10 m; the flow velocity was computed by dividing the distance measurement by the time (Gordon, McMahon, & Finlayson, Citation1994). We determined turbidity in nephelometric turbidity unit (NTU) and pH with the Portable Turbidity meter model WGZ-B and pH meter (HANNA HI 9828 multi-probe meter manufactured by HANNA instruments), respectively. On the other hand, we took electrical conductivity (EC), and total dissolved solids (TDS) readings with Conductivity meter DDSJ-308A. Dissolved oxygen (DO) in water, five days biochemical oxygen demand (BOD5) and nutrient variables, including phosphate and nitrate were analyzed following APHA (American Public Health Association) (Citation1998).
Sampling of aquatic insects
Aquatic insects sample collections were carried out early in the morning between the month of January and February then April to July 2018. Sampling was not done in March 2018 due to logistic problems. Kick net method techniques were employed for the collection of aquatic insects. The Kick net used in this collection is a square-shaped instrument with a long metal handle (Merritt & Cummins, Citation1998).
During sampling, the kick-net was inserted in the river at the littoral zone and approached by walking upstream to disturb the substrate for sample collection. Five kicks were done in each station on every sampling expedition. After kicking, each sample was placed inside a white enamel tray for sorting using forceps.
Identification and preservation of the samples
The specimens collected in the field were preserved in 10% formalin before being taken to the laboratory for identification and enumeration. In the laboratory, samples were placed in a slide and viewed using a binocular microscope for proper identification, following (Javier, David, & Rafael, Citation2011) pictorial guide. After which, voucher samples of the aquatic insects were preserved in 40% formalin for future reference.
Data analyses
We computed the descriptive statistics of physicochemical parameters, including range, mean and standard error for each station, and two-way analysis of variance (ANOVA) was used to test the level of significant differences between stations and months. We then further used post hoc (honestly significance difference; HSD) test to determine stations that differed from each other. The descriptive statistics, ANOVA and HSD, were calculated using the PAST statistical package (Hammer, Harper, & Ryan, Citation2001). The Structural-assemblage of aquatic insects was presented in a table. We used ANOVA to test the statistical significant differences in biological metrics, including abundance, number of taxa, Shannon diversity index, evenness, Simpson dominance, Margalef’s index between the sampled stations, and we used the post doc HSD test to indicate metrics that differed statistically, and these tests were conducted in PAST software package (Hammer et al., Citation2001) . Canonical correspondence analysis (CCA) evaluated the relationships between aquatic insect communities and analyzed environmental variables in PAST software package (Hammer et al., Citation2001). We log (x + 1) transformed physical and chemical parameters dataset used for the CCA model to prevent the undue influences of extreme values on the final CCA model. The statistical significance of the CCA model was revealed by a Monte Carlo permutation test at 999 permutations argument (Jckel, Citation1986). Cluster analysis based on Bray–Curtis similarity index ascertained if spatial (stations) or temporal (months) factors primarily influenced aquatic insects’ assemblage distribution in the study area. We run cluster analysis on log (x + 1) transformed aquatic insect abundance data in PAST statistical package (Hammer et al., Citation2001).
Results
Physical and chemical parameters of River Hadejia
Mean and standard error of physical and chemical parameters in River Hadejia are presented in . Two-way analysis of variance (ANOVA) performed showed transparency, depth, and nitrate to be statistically not significantly different (p > .05) among the months sampled. At the same time, air temperature and DO were significantly different (p < .05) in the sampled months. However, physicochemical parameters mean values did not differ among the stations sampled (p > .05). Mean electrical conductivity was lowest in Station 3 (104.3 ± 8.04 µS/cm). The DO and BOD5 values portray a relatively perturbed water state. The DO value ranged from 1.1 to 5.7 mg/l with the highest mean value (3.44 ± 0.8 mg/l) and BOD5 (1.04 ± 0.3mg/l) recorded in Station 3. Nutrients (nitrate and phosphate) were relatively low during the study period. The river was slightly alkaline, as revealed by the mean pH value of 7.27, 7.77, and 7.82 for Stations 1, 2, and 3, respectively. Except for DO and BOD5, all the parameters were within the permissible limit in the Nigeria Federal Environmental Protection Agency (FEPA) and Standard Organization of Nigeria (SON).
Table 1. Summary of physical and chemical parameters measured at the study stations of River Hadejia
Aquatic insect community structure of River Hadejia
Four orders of aquatic insects belonging to 11 families and taxa were recorded during the entire study period. Dytiscidae (Dytiscus sp.) was the most abundant taxon in the study area. Pollution-sensitive species were sparingly represented by Ephemeroptera taxa with two representative taxa (Baetidae and Ephemerellidae). A total of 44, 37, and 35 individuals of aquatic insects were recorded in stations 1, 2, and 3 in the river. Generally, a sparse distribution and abundance of aquatic insects were noticed in the River Hadejia.
Ecological indices of aquatic insect in River Hadejia
Mean diversity indices of aquatic insects are presented in . Mean taxa (number of species), Simpson dominance, Shannon Weiner index, and Margalef index (taxa richness) were significantly higher in Station 3. Stations 1, 2, and 3 showed no significant difference in the mean value of Evenness. Abundance (number of individuals) was higher in station 1 (7.83 ± 2.96). Post hoc test performed for all the diversity indices were not significantly different between stations (p > .05).
Table 2. Structural assemblage of aquatic insects in River Hadejia
Relationship between aquatic insects and physical and chemical parameters in River Hadejia
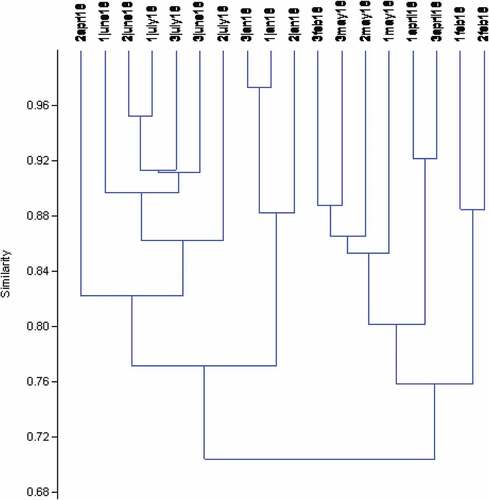
The CCA revealed a little or no relationship between the physical and chemical parameters and the aquatic insects. However, the first canonical axis explained over 70% of the variation in the aquatic insect’s data set, indicating a good ordination model. The eigenvalues for axes 1 and 2 were 0.126 and 0.054, respectively (). The Monte Carlo permutation test performed on the first two canonical axes showed no significant difference (p > .05). Dysticus sp. and Platyenemidae were associated to axis 1, while the other remaining aquatic insects were associated to axis 2 except Naucoris sp. and Hydrophilus sp., which were located at the center of the CCA triplot (). Gyrinus sp. was positively affected by increased water depth. Biochemical oxygen demand had a relatively strong correlation with Nepa sp. From the CCA triplot, Stations 1, 2 and 3 only had Dytiscus sp., Naucoris sp. and Nepa sp. in common, while the other aquatic insects were not linked to any station specifically as seen in the scattered distribution of the biota in the triplot (). Generally, no physical and chemical parameters showed correlation with the aquatic insects collected during the study except for TDS that shows a slight association with Noterus sp. and BOD5 slightly affected the distribution of Ephemerella sp. and Nepa sp. Cluster analysis indicated that aquatic insects clustering were mainly influenced by month rather than by stations, with insects collected from the same month more closely associated than those collected from stations ().
Table 3. Weighted intraset correlations of physicochemical parameters with the first two axes of canonical correspondence analysis (CCA) in River Hadejia, Jigawa State, Nigeria
Figure 2. Triplot of the first and second CCA axes of aquatic insect taxa, physicochemical variables and the sampling stations of River Hadejia, Jigawa State, Nigeria. Aquatic insect abbreviation: Dry (Dryopidae), Not (Noterus sp.), Dyt (Dytiscus sp.), Gyr (Gyrinus sp.), Hyd (Hydrophilus sp.), Npa (Nepa sp.), Nau (Naucoris sp.), Bet (Baetis sp.), Eph (Ephemerella sp.), Lib (Libellula sp.), Pla (Platyenemidae).
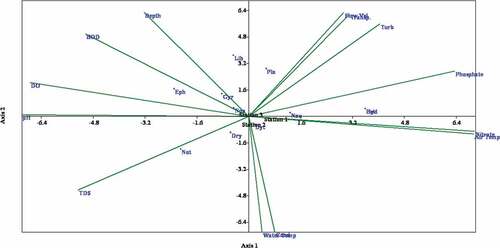
Figure 3. Dendrogram derived from cluster analysis (Bray Curtis similarity index) of log (x + 1) transformed Aquatic insect data in River Hadejia during the study period. Jan for January, Feb for February Apr for April, May, June and July. Numbers 1, 2 and 3 attach to the month represents sampled stations.
Discussion
Physical and chemical parameters
Deteriorating physical and chemical parameters in a given water body are reported to have a debilitating effect on aquatic macroinvertebrates distribution and abundance (Arimoro et al., Citation2015; Sundermann, Gerhardt, Kappes, & Haase, Citation2013). The high EC and BOD5 in the present study indicate a distressed watercourse. This, no doubt, may be occasioned by the incessant human influences on the river. Earlier studies elsewhere in southern Nigeria have reported a similar occurrence in river courses and its catchments (Andem, Okorafor, Eyo, & Ekpo, Citation2014; Arimoro et al., Citation2015). Study in selected rivers in Tanzania revealed a perturbed water quality due to the debilitating effect of anthropogenic activities on the water systems (Kaaya et al. Citation2015). Reduced DO concentration in all the study stations sampled is also a pointer to the river’s heavy alteration. This may be caused by the influx of fertilizer runoff from nearby farmlands as northern Nigeria is known for extensive farming activities for commercial purposes. Most farmers in the area use fertilizer and other chemicals to cultivate their crops. Generally, it may be concluded that the deteriorating water state of River Hadejia is as a result of uncontrolled human disturbance on the river due to unenforced or poorly enforced regulations guiding the water bodies in Nigeria. Hence, the ravaging state of rivers and other water bodies in Nigeria.
Structural-assemblage of aquatic insects
Aquatic insects in various quarters have been used as biomonitoring tools in assessing the ecological health of water bodies (Barman & Gupta, Citation2015; Edegbene, Arimoro, Odoh, & Ogidiaka, Citation2015). In this study, Dytiscidae (Dytiscus sp.) was the most predominant species and well represented in the three stations sampled. This may be hinged on favorable environmental or other conditions that enhance this group of aquatic insects in River Hadejia. Studies have suggested structural-assemblage changes due to geomorphological factors and other instream destruction of the physical habitat, as factors that contribute to the abundance and distribution of some macroinvertebrates (Barman & Gupta, Citation2015; Selvakumar, Sivaramakrishnan, Janarthanan, Arumugam, & Arunachalam, Citation2014). Pollution-sensitive insect species were sparingly represented in their present study. This is an indication of perturbed ecological health of the iver. Various authors have reported that Ephemeroptera, Plecoptera and Trichoptera (EPT) indicate moderately disturbed to clean water (Adakole & Anunne, Citation2003; Akamagwuna et al. Citation2019b) depending on their species composition and abundance.
Hemiptera was the second most abundant aquatic insect in the study area. Studies elsewhere have reported a similar trend in the preponderance of this group of macroinvertebrates (Huang, Lock, Chi Dang, De Pauw, & Goethals, Citation2010; Naranjo, Riviaux, Moreira, & Court, Citation2010; Takhelmayum, Gupta, & Singh, Citation2013). This they ascribed to the ability of hemipteran to utilize atmospheric oxygen as they skate on the surface of the water in the face of deteriorating DO concentration. This may be why Hemiptera was fairly represented in River Hadejia despite the fact that DO concentration was very low. It can be inferred from the structural assemblage of aquatic insects in River Hadejia that the water is fast deteriorating, judging from the weak structural assemblage of the insects.
Ecological indices and diversity of aquatic insects
Diversity indices performed confirmed the reaches of the river studied to be perturbed. The mean Margalef index (taxa richness) for the three stations was less than 3. It has been earlier proclaimed by Lenat, Smock, and Penrose (Citation1980) that the Margalef index value greater than 3 indicates clean water condition while a value less than 1 portrays polluted water. In the present study, the taxa richness values for the three stations are less than 1, further confirming the devastating effect of deteriorating water state on the aquatic biota and the ecological health condition of the river. Recently, a study in a dam in Northern Nigeria reported a closely similar trend of taxa richness (Edegbene, Citation2020). This was reported to be due to poor environmental factors in the dam occasioned by the menace posed by Typha grass and other human activities.
Relationship between aquatic insects and environmental variables
Canonical correspondence analysis (CCA) constructed for this study revealed a little or no association between the aquatic insects and the physical and chemical parameters. The eigenvalue in axes 1 and 2 derived from CCA triplot was less than 1.0. Eigenvalues associated with each axis equal the correlation coefficient between species and stations scores (Gauch, Citation1982; Pielou, Citation1984). Thus, an eigenvalue close to 1 represents a high degree of correlation between species and stations or any other variable and an eigenvalue close to zero indicates little correlation (Palmer, Citation1993). For instance, in this present study, the CCA triplot showed that axis 1 had an eigenvalue of 0.126 while axis 2 was 0.054. This indicates a very low correlation between the aquatic insects, the physical and chemical variables, and the sampled stations. Axis 2 in the CCA triplot was weakly associated with aquatic insects, as revealed from the near-zero eigenvalue. We suggest Nepa sp. as an indicator of deteriorating ecological health conditions of freshwater systems as they were influenced by increased BOD5 concentration. At the same time, Gyrinus sp. was positively affected by increased water depth. Hence, Gyrinus sp. may be affirmed to be a deep water dweller.
Conclusion and recommendation
This study serves as a baseline survey on the use of aquatic insects in assessing the health of River Hadejia. The river’s ecological health has been compromised due to the various factors listed above, ranging from poor environmental variables to poor structural assemblage of aquatic insects. For example, air temperature and DO differed significantly between months sampled. Season differences were also more influential in affecting the water quality of the Hadejia rivereine system than site spatial differences. Sensitive insects’ species of the orders Ephemeroptera Plecoptera and Trichoptera (EPT) were poorly represented in the study area, with Baetidae and Ephemerellidae (order Ephemeroptera) being the predominant EPTs, further indicating poor water quality conditions. Hence, it can be concluded that the influence of human activities has a debilitating effect on the health of River Hadejia. However, we recommend that further studies should be conducted which will involvemultiple sites and rivers within the Hadejia emirate and its envrion to confirm the results of this study and to understand better the effects of pollution on the functionality of river systems within the emirate. We further recommend that more stringent regulations to control human pressure on the river systems within the studied area to enable surface waters in the area to sustain the provision of desired and valued ecosystem services.
Author Contributions
AOE conceptualized and designed the study. FG and AOE carried out the field sampling, sorting, and identification on monthly basis. FG and AOE performed the data analyses. FG, EO and AOE did the literature review search. AOE, FG and EO wrote the initial draft of the manuscript. FCA drew the study area map. AOE, FCA and KHN reviewed and polished the final manuscript. AOE supervised the entire research project work. All authors read and approved the final manuscript.
Acknowledgments
We acknowledge the field assistance and proofreading of the first manuscript by Mrs. Edegbene Ovie Tega Treasure.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abebe, B., Taffere, A., Dermake, K., Worku, L., Helmet, K., & Ludwig, T. (2009). Comparative study of severe water pollution: Case study of the Kebena and Akaki Rivers in Addis Ababa, Ethiopia. Ecological Indicators, 9(2), 381–392. doi:10.1016/j.ecolind.2008.05.001
- Abowei, J. F. N., & Sikoki, F. D. (2005). Water pollution management and control. Calabar, Nigeria: Doubletrust.
- Abubakar, B. H. (2009). Weather focus in Hadejia. Liberty printing press, Hadeja, Jigawa State Nigeria.
- Adakole, J. A., & Anunne, P. A. (2003). Benthic macroinvertebrates as indicators of environmental quality of an urban stream in Zaria, Northern Nigeria. Journal of Aquatic Sciences, 18(2), 85–92.
- Ahmed, S. D., Agodzo, S. K., Adjei, K. A., Deinmodei, M., & Ameso, V. C. (2018). Preliminary investigation of flooding problems and the occurrence of kidney disease around Hadejia-Nguru wetlands, Nigeria and the need for an ecohydrology solution. Ecohydrology and Hydrobiology, 18(2), 212–224. doi:10.1016/j.ecohyd.2017.11.005
- Akamagwuna, F. C., Mensah, P. K., Nnadozie, C. F., & Odume, O. N. (2019a). Evaluating the responses of taxa in the orders Ephemeroptera, Plecoptera and Trichoptera (EPT) to sediment stress in the Tsitsa River and its tributaries, Eastern Cape, South Africa. Environmental Monitoring and Assessment, 191, 11. doi:10.1007/s10661-019-7846-9
- Akamagwuna, F. C., Mensah, P. K., Nnadozie, C. F., & Odume, O. N. (2019b). Traits-based responses of Ephemeroptera, Plecoptera and Trichoptera to sediment stress in the Tsitsa River and its tributaries, Eastern Cape, South Africa. River Research and Applications, 35(7), 999–1012. doi:10.1002/rra.3458
- Al-Zankana, A. F. A., Matheson, T., & Harper, D. M. (2020). How strong is the evidence – Based on macroinvertebrate community responses – That river restoration works? Ecohydrology and Hydrobiology, 20(2), 196–214. doi:10.1016/j.ecohyd.2019.11.001
- Andem, A. B., Okorafor, K. A., Eyo, V. O., & Ekpo, P. B. (2014). Ecological impact assessment and limnological characterization in the intertidal region of Calabar River using benthic macroinvertebrates as bioindicator organisms. International Journal of Fisheries and Aquatic Studies, 1(2), 8–14.
- APHA (American Public Heath Association). (1998). Standard methods for the examination of water and wastewater, WEF and AWWA (20th Edition ed.). USA.
- Arimoro, F. O., Odume, O. N., Uhunoma, S. I., & Edegbene, A. O. (2015). Anthropogenic impact on water chemistry and benthic macroinvertebrate associated changes in a southern Nigeria stream. Environmental Monitonitoring and Assessment, 187(2), 1–14.
- Asonye, C. C., Okolie, N. P., Okenwa, E. E., & Iwuanyanwu, U. G. (2007). Some physic-chemical characteristics and heavy metal profiles of Nigerian rivers, streams and waterways. African Journal of Biotechnology, 6(5), 617–624.
- Barman, B., & Gupta, S. (2015). Aquatic insects as bio-indicator of water quality-A study on Bakuamari stream, Chakras hila wildlife sanctuary, Assam, North East India. Journal of Entomology and Zoology Studies, 3(3), 178–186.
- BirdLife International. (2016). Important Bird and Biodiversity Area factsheet: Hadejia- Nguru wetlands. http://www.birdlife.orgon. 17/04/2018.
- Edegbene, A. O. (2020). Potential menace posed by invasive grass and water quality deterioration on macroinvertebrates structural distribution in a dam in North- Western Nigeria. Water Science, 34(1), 75–84. doi:10.1080/11104929.2020.1751918
- Edegbene, A. O., Arimoro, F. O., Odoh, O., & Ogidiaka, E. (2015). Effect of anthropogenicity on the composition and diversity of aquatic insects of a municipal river in North Central Nigeria. Bioscience Research in Today’s Word, 1(1), 69–80.
- Edegbene, A. O., Arimoro, F. O., & Odume, O. N. (2019). Developing and applying a macroinvertebrate-based multimetric index for urban rivers in the Niger Delta, Nigeria. Ecology and Evolution, 9(22), 12869–12885. doi:10.1002/ece3.5769
- Eriksen, T. E., Brittain, J. E., Søli, G., Jacobsen, D., Goethals, P., & Friberg, N. (2021). A global perspective on the application of riverine macroinvertebrates as biological indicators in Africa, South-Central America, Mexico and Southern Asia. Ecological Indicators, 126, 107609. doi:10.1016/j.ecolind.2021.107609
- Feio, M. J., Hughes, R. M., Callisto, M., Nichols, S. J., Odume, O. N., Quintella, B. R., … Yates, A. G. (2021). The biological assessment and rehabilitation of the World’s Rivers: An overview. Water, 13(3), 371. doi:10.3390/w13030371
- FEPA (Federal Environmental Protection Agency). (1991). Guidelines and standards for environmental pollution control in Nigeria. Abuja: FEPA.
- Firmiano, K. R., Castro, D. M. P., Linares, M. S., & Callisto, M. (2021). Functional responses of aquatic invertebrates to anthropogenic stressors in riparian zones of Neotropical savanna streams. Science of the Total Environment, 753, 141865. doi:10.1016/j.scitotenv.2020.141865
- Garba, A., Ekanem, E. O., & Garba, I. H. (2017). Quality assessment of groundwater from Hadejia local government area of Jigawa State, Nigeria. Bayero Journal of Pure and Applied Sciences, 9(2), 258. doi:10.4314/bajopas.v9i2.44
- Gauch, H. G. (1982). Multivariate analysis and community structure. Cambridge, England: Cambridge University Press.
- Gieswein, A., Hering, D., & Lorens, A. W. (2019). Development and validation of a macroinvertebrate-based biomonitoring tool to assess fine sediment impact in small mountain streams. Science of the Total Environment, 652, 1290–1301. doi:10.1016/j.scitotenv.2018.10.180
- Gordon, N. D., McMahon, T. A., & Finlayson, B. L. (1994). Stream Hydrology, an introduction for Ecologists. New York. John Wiley & Sons Ltd.
- Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4(1), 9. Accessed 1 July 2015. http://palaeo-electronica.org/2001_1/past/issue1_01.htm.
- Huang, T. H., Lock, K., Chi Dang, K., De Pauw, N., & Goethals, P. L. M. (2010). Spatial and temporal patterns of macroinvertebrate communities in the Du river Basin in Northern Vietnam. Journal of Freshwater Ecology, 25(4), 637–647. doi:10.1080/02705060.2010.9664413
- Javier, O., David, G., & Rafael, M. Eds. (2011). Identification guide of freshwater macroinvertebrates of Spain. 1. 10.1007/978-94-007-1554-7_1 © Springer Science+Business Media B.V.
- Jckel, K. (1986). Finite sample properties and asymptotic efficiency of Monte Carlo tests. Journal of Applied Econometric, 14, 85–118.
- Kaaya, L. T., Day, J. A., & Dallas, H. F. (2015). Tanzania river scoring system (TARISS): a macroinvertebrate-based biotic index for rapid bioassessment or rivers. African Journal of Aquatic Science, 40(2), 109–117.
- Lenat, D. R., Smock, L. A., & Penrose, D. L. (1980). Use of benthic macroinvertebrates as indicators of environmental quality. In Biological monitoring for environmental effects, lexinton books (pp. 7–114). Toronto, Canada.
- Lubanga, H. L., Manyala, J. O., Siati, A., Yegon, M. J., & Masese, F. O. (2021). Spatial variability in water quality and macroinvertebrate assemblages across a disturbance gradient in the Mara River. Ecohydrology & Hydrobiology, 21(4), 718–730. doi:10.1016/j.ecohyd.2021.03.001
- Merritt, R. W., & Cummins, K. W. (1998). An introduction to the aquatic insects of North America (3rd ed.). IOWA, Dubuque: Kendall-Hunt.
- Naranjo, C., Riviaux, S. M., Moreira, F. F. F., & Court, R. C. (2010). Taxonomy and distribution of aquatic and semiaquatic Heteroptera (Insecta) from Cuba. International Journal of Tropical Biology, 58, 897–907.
- Palmer, M. W. (1993). Putting things in even better order: The advantages of canonical correspondence analysis. Ecology, 74(8), 2215–2230. doi:10.2307/1939575
- Parienté, W. (2017). Urbanisation in Sub-Saharan Africa and challenges of access to basic services. Journal of Demographic Economics, 83(1), 31–39. doi:10.1017/dem.2017.3
- Pielou, E. C. (1984). The interpretation of ecological data: A primer on classification and ordination. New York, USA: Wiley, New York.
- Selvakumar, C., Sivaramakrishnan, K. G., Janarthanan, S., Arumugam, M., & Arunachalam, M. (2014). Impact of riparian land landuse patterns on Ephemeroptera community structure in river basins of the southern Western Ghats, India. Knowledge and Management of Aquatic Ecosystem, 412(412), 11. doi:10.1051/kmae/2013093
- Sundermann, A., Gerhardt, M., Kappes, H., & Haase, P. (2013). Stressor prioritisation in riverine ecosystems: Which environmental factors shape benthic invertebrate assemblage metrics? Ecological Indicators, 27, 83–96. doi:10.1016/j.ecolind.2012.12.003
- Takhelmayum, K., Gupta, S., & Singh, N. R. (2013). Diversity and density of aquatic insects in the lower reach of River Moirang, Manipur, North East India. Proceedings of the National Academy of Sciences, India Section B. Biological Sciences, 83(4), 575–584.
- Umar, D. A., Ramli, M. F., Aris, A. Z., Jamil, N. R., & Abdulkareem, J. H. (2018). Runoff irregularities, trends, and variations in tropical semi-arid river catchment. Journal of Hydrology: Regional Studies, 19(October), 335–348. doi:10.1016/j.ejrh.2018.10.008
- Umara, D. A., Ramlib, M. F., Arisc, A. Z., Jamilb, N. R., & Tukur, A. I. (2019). Surface water resources management along Hadejia River basin, northwestern Nigeria. H2Open Journal, 2(1), 184–199. doi:10.2166/H2OJ.2019.010
- Yule, C. M., & Yong, H. S. (2004). Freshwater invertebrates of the Malaysian region. Malaysia: Academy of Sciences Malaysia.
- Yung-Chul, J., Nan-Young, K., Sang-Hun, K., Young-Seuk, P., Dong-Soo, K., & Soon-Jin, H. (2016). Spatial-distribution of benthic macroinvertebrate assemblages in relation to environmental variables in Korean Nationwide stream. Water, 8(27), 1–20. doi:10.3390/w/8010027