ABSTRACT
Chronic stress drives cancer progression, but the routes of metastasis are unclear. We recently demonstrated that chronic stress activates a neural-inflammatory signaling axis to remodel lymphatic vasculature and increase lymph flow. This unanticipated crosstalk between stress and the lymphatic system provides pathways of tumor cell dissemination and accelerates metastasis.
To enhance alertness and rapidly mobilize a protective response to threat, our bodies trigger a stress response that includes activation of the sympathetic nervous system (SNS).Citation1 When acutely activated, this “fight-or-flight” response is beneficial for survival. However, prolonged or chronic stress can be detrimental to health.Citation2-5 In particular, a growing body of evidence suggests that stressful events promote metastasis and lead to poor cancer survival,Citation2,3,5,6 although the routes of tumor cell dissemination are not clear.Citation2,5 Tumor cell invasion into the lymphatic system is linked to poor prognosis in solid tumors.Citation7 The lymphatic system is innervated by sympathetic nervous system (SNS) fibers; however, the effects of stress on lymphatic function and the consequences of this effect on cancer progression have yet to be explored. Our recent publication in Nature Communications demonstrated that chronic stress restructures lymphatic vessels to accelerate the dissemination of tumor cells. This work identifies lymphatic vessels as an additional pathway of tumor cell escape that might be targeted to effectively block the adverse effects of stress.Citation8
Using an orthotopic mouse model of breast cancer, physiological SNS signaling was activated using a restraint stress paradigm whereby inescapable confinement induces escape-avoidance behavior and elevates levels of stress hormones, including norepinephrine.Citation2,5 Chronic stress increased the density of lymphatic vessels within tumors, as assessed by immunohistochemical staining. Chronic stress also increased the diameter of collecting lymphatic vessels that drain primary tumors toward distant lymph nodes. This effect was maintained even 2 weeks after stress ceased, suggesting a stable, increased capacity for tumor cell dissemination via lymphatic routes in stressed animals. Intravital microscopy to detect disseminated tumor cells confirmed the functionality of these tumor-draining lymphatic vessels.Citation8 Additionally, longitudinal in vivo bioluminescence imaging confirmed that stress-induced lymphatic remodeling was associated with increased metastasis to lymph nodes and distant organs, consistent with tumor lymphatic vessel density and lymphatic vessel dilation having key roles in lymphogenous tumor cell dissemination.Citation9
Intravital microscopy confirmed that the lymphatic system is innervated by the SNS, with catecholaminergic TH+ (tyrosine hydroxylase) fibers present throughout the lymph node capsule and parenchyma. This direct link between fibers of the SNS and the lymphatic system raised the possibility that, in addition to restructuring lymphatic vasculature, SNS signaling could also affect lymph flow. Therefore, we used nuclear lymphoscintigraphy to track the effect of sympathectomy by neuraxial anesthesia on lymph flow in the lower limbs of a consenting patient. Tracking lymph drainage of a radiolabeled tracer revealed that sympathetic blockade reduced lymph flow by 80%. To investigate whether, conversely, activating SNS signaling would promote lymph flow, we developed a method that directly visualizes lymph flow in vivo by tracking the transit of fluorescently tagged nanospheres through lymphatic vessels of anaesthetized mice. Intravenous administration of the stress neurotransmitter norepinephrine significantly increased lymph flow through collecting vessels, demonstrating that SNS signaling is a potent regulator of lymph flow.Citation8 Inhibition of norepinephrine signaling through β-adrenoceptors using the β-blocker propranolol stopped stress-induced lymphatic remodeling and blocked metastasis to lymph nodes, identifying a pharmacological strategy for intervention in stress-induced lymphogenous dissemination.
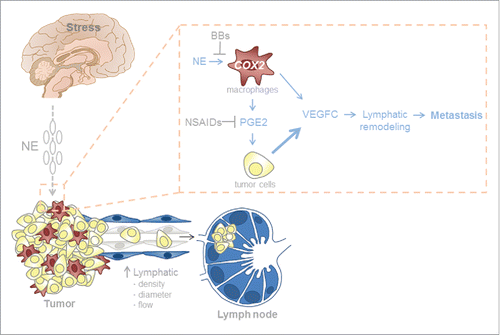
Gene expression analysis of tumors revealed that chronic stress increased expression of the lymphatic vascular endothelial growth factor C (VEGFC) in primary tumors.Citation8 VEGFC promotes tumor lymphangiogenesis and metastasis to lymph nodes. Knockdown of VEGFC in tumor cells reduced stress-enhanced lymph vessel density and prevented lymphogenous dissemination, demonstrating a key role for tumor cell-derived VEGFC in the effects of stress on lymphatic remodeling and metastasis. Further analyses found that crosstalk between tumor cells and macrophages is required for these changes to the tumor lymphatic vasculature. We found that macrophages respond to stress by releasing prostaglandins, which elevate tumor cell production of VEGFC leading to vascular remodeling and metastasis to lymph nodes. Notably, inhibition of either inflammatory signaling with a cyclooxygenase-2 (COX2) inhibitor or macrophage activity with a small molecule inhibitor of colony stimulating factor 1 blocked stress-induced lymphatic metastasis.Citation8
Together, these data demonstrate that the coordinated regulation of the SNS response to stress and lymphatic function—which may have provided an evolutionary advantage to mobilize immune surveillance and direct an immune response during times of threat—can be hijacked by tumors to promote cancer spread.
Implications
As the impact of neural signaling on cancer becomes clearer, it is imperative that stress-sensitive steps in cancer progression are identified for the development of targeted interventions. The findings presented here suggest that targeting either neural or inflammatory signaling activated by stress may limit metastatic dissemination and cancer-related mortality (). This is in line with recent retrospective epidemiologic studies linking β-blocker use to improved cancer outcomes.Citation10 We now demonstrate that β-blocker use in cancer patients is linked to reduced tumor cell dissemination to lymph nodes.Citation8 These findings provide a mechanistic rationale for ongoing clinical trials using β-blockers as a possible treatment option for patients with cancer.
Figure 1. Chronic stress promotes the dissemination of cancer cells by remodeling lymphatic vasculature. Activation of the sympathetic nervous system increases levels of catecholamines, norepinephrine (NE), and/or epinephrine (E). Tumor-associated macrophages respond to NE/E by secreting inflammatory molecules such as prostaglandin E2 (PGE2), which drive the production of vascular endothelial growth factor C (VEGFC) in tumor cells. Tumor cell-derived VEGFC then facilitates lymphatic remodeling and metastasis. Various points in this signaling cascade can be targeted using drugs such as β-blockers (BBs) or non-steroidal anti-inflammatory drugs (NSAIDs).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The research described here was funded by the Australian National Health and Medical Research Council (1008865 and 1053535), the Australian Research Council (LE110100125), the National Cancer Institute (CA160890), the Australian and New Zealand College of Anaesthetists (N13/002), the Co-operative Research Center for Cancer Therapeutics and the National Breast Cancer Foundation.
References
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 2007; 87:873-904; PMID:17615391; http://dx.doi.org/10.1152/physrev.00041.2006
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 2006; 12:939-44; PMID:16862152; http://dx.doi.org/10.1038/nm1447
- Kim-Fuchs C, Le CP, Pimentel MA, Shackleford D, Ferrari D, Angst E, Hollande F, Sloan EK. Chronic stress accelerates pancreatic cancer growth and invasion: A critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun 2014; 40:40-7; PMID:24650449; http://dx.doi.org/10.1016/j.bbi.2014.02.019
- Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS. Autonomic nerve development contributes to prostate cancer progression. Science 2013; 341:1236361; PMID:23846904; http://dx.doi.org/10.1126/science.1236361
- Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 2010; 70:7042-52; PMID:20823155; http://dx.doi.org/10.1158/0008-5472.CAN-10-0522
- Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol 2008; 5:466-75; PMID:18493231; http://dx.doi.org/10.1038/ncponc1134
- Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347:1233-41; PMID:12393820; http://dx.doi.org/10.1056/NEJMoa022152
- Le CP, Nowell CJ, Kim-Fuchs C, Botteri E, Hiller JG, Ismail H, Pimentel MA, Chai MG, Karnezis T, Rotmensz N, et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun 2016; 7:10634; PMID:26925549; http://dx.doi.org/10.1038/ncomms10634
- Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, Ardipradja K, Zhang YF, Williams SP, Farnsworth RH, Chai MG, et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer cell 2012; 21:181-95; PMID:22340592; http://dx.doi.org/10.1016/j.ccr.2011.12.026
- Le CP, Karnezis T, Achen MG, Stacker SA, Sloan EK. Lymphovascular and neural regulation of metastasis: shared tumour signalling pathways and novel therapeutic approaches. Best Pract Res Clin Anaesthesiol 2013; 27:409-25; PMID:24267548; http://dx.doi.org/10.1016/j.bpa.2013.10.008
