ABSTRACT
Tumor niche extracellular matrix stiffening and tumor cell metabolic reprogramming are two fundamental mediators of tumor progression. We recently elucidated a mechanistic interconnection between mechanotransduction and tumor metabolic rewiring in cancer. We demonstrated a stiffness-dependent amino acid crosstalk between stromal and cancer cells that fuels tumor progression and metastatic spreading.
Tumor progression and consequent metastasis account for the majority of demises associated with tumor development. Undoubtedly, genetic modifications in tumor cells initiate and drive malignancy; Cancers; however, progress within a dynamically-evolving tumor ecosystem which, among other factors, requires extracellular matrix (ECM). Cancer-dependent remodeling of the ECM virtually modulates every behavioral facets of both tumor cells and cancer-associated fibroblasts (CAFs), including sustained cell proliferation and initiation of invasion.Citation1 Understanding how the cell-ECM molecular interactions mediate cancer development and metastatic spreading is essential to identify novel potential therapeutic targets for treatment of patients.
Fundamental aspects of cell behavior in living organisms – morphogenesis, collective migration and self-organization – are not solely genetically driven, but also consist of properties emerging from interconnections established by the cell within a tissue network. Furthermore, as in cancer, most acquired conditions, are preceded or accompanied by corruption of the tissue ecosystem, which fuels aberrant cell behaviors. The highly related transcriptional regulators Yes associated protein 1 (YAP1 also known as YAP) and WW domain containing transcription regulator 1 (WWTR1 best known as TAZ) have recently emerged as fundamental sensors by which cells decipher the structural and architectural features of their tissue microenvironment through mechanotransduction-dependent signalingCitation2 (i.e., the processes that enable cells to sense and adapt to external mechanical forces). Thus, YAP and TAZ function as central regulators of cellular proliferation, survival and polarity, specifically in development and cancer progression.
Warburg metabolism, a chronic shift in energy production, from mitochondrial oxidative phosphorylation to glycolysis, has been described as a pathogenic driver of tumor progression. Increasing evidence suggests a central connection of mechanotransduction – including the YAP/TAZ pathway – with cellular metabolism, processes related to glucose consumption and Warburg metabolism.Citation3,Citation4 However, increased glycolysis alone is insufficient to meet the total metabolic demands of proliferating cells. Yet, critical insights regarding any additional metabolic reprogramming initiated by mechanical cues in solid tumors were lacking.
We hypothesized that mechanical stimuli from the tumor niche provide crucial molecular signals to guide tumor cells and CAF in capturing nutrients to support their metabolic needs (). Using a co-culture organoid system, orthotopic syngenic mouse models of carcinoma, patient-derived xenograft mouse model of carcinoma, and tumor biopsies from patients, we defined a critical molecular connection between mechanoactivation of YAP/TAZ by tumor niche stiffening and up-regulation of the key metabolic enzymes lactate dehydrogenase A (LDHA) and glutaminase (GLS), and the amino acid transporter solute carrier family 1, member 3 (SLC1A3). We found that such metabolic reprogramming coordinates glycolysis and non-essential amino acid exchange within the tumor niche. Inhibiting either matrix stiffening, cell mechanics or amino acid exchange prevented the metabolic reprogramming, which blunted tumor progression and metastasis in vivo.Citation5 These results improve the basic understanding of the deregulated metabolic axis within the tumor by revealing critical links between glucose metabolism and amino acid exchange through a shared hierarchy of regulation via mechanotransduction (). Altogether, these results place glutaminolysis and aspartate/glutamate exchange as a central mediator of the action of the extracellular environment on tumor cell functions and provide evidence that targeting the YAP/TAZ–GLS-SLC1A3 axis will act on both tumor and stroma cells to prevent tumor growth and metastatic spreading.
Figure 1. Mechanical regulation of the metabolic network that drives tumor progression by interfacing cancer and stromal cells. Extracellular matrix (ECM) remodeling by Carcinoma-Associated Fibroblast (CAF) within the tumor microenvironment promotes matrix stiffening. Stiffer matrix, in turn, favors epithelial cancer cell proliferation and metastatic spreading (A). Mechanical activation of the YAP/TAZ-dependent transcriptional program in cells results in increase of the glutaminase (GLS), lactate dehydrogenase A (LDHA) and aspartate/glutamate transporter SLC1A3 genes, thus coordinates glycolysis and amino acid availability within the tumor niche. Mechanistically, in epithelial cancer cells, this mechanical response results in glutamate accumulation accompanied by a defect of the TCA cycle and an impaired production of aspartate. In stromal fibroblasts, the mechanically regulated accumulation of glutamate leads to production of aspartate and control of acto-myosin-dependent ECM remodeling. Finally, the aspartate produced by the stromal fibroblasts is released within the tumor microenvironment and uptaken by the tumor cells to supply their proliferative needs. While the glutamate produced by the cancer cell is released within the tumor microenvironment and uptaken by the stromal fibroblasts to balance their redox level which appear key for their remodeling activity. In summary, mechanical activation of YAP/TAZ in the tumor niche coordinate cancer cells proliferation and stromal fibroblasts-dependent ECM remodeling with their energetic and biosynthetic requirements to force metastatic spreading (B). SCC: squamous cell carcinoma.
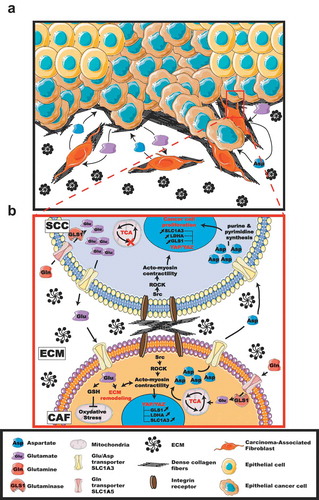
While context-specific studies demonstrated metabolic cooperation between cancer cells and CAF within tumors,Citation6,Citation7 the role of mechanotransduction in such a process remained obscure. To insight this regulatory pathway, we adopted an approach where differential metabolic transformations in a reactive stroma (a stiff ECM) versus a normal stroma (a soft ECM) were identified. Importantly, while our findings report focuses mainly on glutaminolysis/anaplerotic pathways, our experiments focused on only a few metabolites and biochemical pathways. Thus, the true breadth of influence by the tumor niche stiffness in metabolic reprogramming may be even more broad. Moreover, because we identified SCC-CAF amino acid crosstalk as a key mechanism to sustain tumor progression in response to ECM stiffness, it will be crucial to determine whether similar metabolic cooperation occurs between other stromal cells within the tumor.
In tumor niche contexts, YAP/TAZ has been reported to control cell proliferation and contractility in response to the mechanical cues from the environment.Citation2 Here, we define the mechanotransduction cascade mediated by the YAP/TAZ co-transcription factor as a lynchpin between biophysical cues and the metabolic adaptations required for growth in stiff tumor microenvironments. This reciprocity among YAP/TAZ with upstream and downstream metabolic cues suggests an adjustable feedback-driven property that may be partly responsible for individualized “tuning” of the metabolic program within the tumor.Citation4,Citation8-Citation10 Resolution at the single-cell level and development of single-cell metabolomics will be critically important to decipher the metabolic architecture of tumors.
The mechanical rewiring of metabolism of tumor cells makes aspartate a crucial determinant for proliferation.Citation5,Citation8 Under the enzymatic activity of GLS, glutamine is transformed into glutamate to fuel the tricarboxylic acid cycle (TCA) cycle through anaplerosis. Interestingly, in response to ECM stiffening, glutamine incorporation into the cancer cells is increased, together with production of glutamate, but it does not feed the TCA cycle, as observed in the stromal fibroblasts. Therefore, ECM stiffening results in glutamate accumulation in cancer cells, and aspartate production in stromal cells. We hypothesize that cancer cells find in their close microenvironment the aspartate produced and secreted by the stromal cells for their own proliferating needs. Therefore, understanding why the anaplerosis fuelling of the TCA cycle is impaired in response to a stiff ECM would provide a better understanding of the molecular impact that the mechanical rewiring exerts on the tumor metabolism.
Although this study demonstrates that mechanotransduction constitutes a key mechanism by which ECM can impose metabolic changes, the mechanisms by which ECM influences other aspects of the tumor cell proliferation and migration are likely to be multifaceted.Citation1 Notably, while ECM stiffness sustains cancer cell proliferation and migration, the ECM biochemical composition also influences tumor cell behavior. Thus, ECM remodeling likely affects tumor cell traits, such as proliferation and migration, through a variety of mechanisms. Our findings focused on matrix stiffness and rich collagen environments, we, therefore, speculate that tissue-specific differences within microenvironmental, biomechanical and biochemical properties confer differential metabolic needs to the cells. Deciphering the interconnection between tumor mechanics and metabolism will offer a cohort of novel diagnostic and therapeutic targets for drug development in cancer and may have broad applications to other human metabolic conditions in which mechanosensitive interactions may serve as key factors of disease.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–1253. doi:10.15252/embr.201439246.
- Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18:758–770. doi:10.1038/nrm.2017.87.
- Bays JL, Campbell HK, Heidema C, Sebbagh M, DeMali KA. Linking E-cadherin mechanotransduction to cell metabolism through force-mediated activation of AMPK. Nat Cell Biol. 2017;19:724–731. doi:10.1038/ncb3450.
- Enzo E, Santinon G, Pocaterra A, Aragona M, Bresolin S, Forcato M, Grifoni D, Pession A, Zanconato F, Guzzo G, et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. Embo J. 2015;34:1349–1370. doi:10.15252/embj.201490379.
- Bertero T, Oldham WM, Grasset EM, Bourget I, Boulter E, Pisano S, Hofman P, Bellvert F, Meneguzzi G, Bulavin DV, et al. Tumor-stroma mechanics coordinate amino acid availability to sustain tumor growth and malignancy. Cell Metab. 2019;29(1):124–140.e10. doi:10.1016/j.cmet.2018.09.012.
- Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature. 2016;536:479–483. doi:10.1038/nature19084.
- Yang L, Achreja A, Yeung TL, Mangala LS, Jiang D, Han C, Baddour J, Marini JC, Ni J, Nakahara R, et al. Targeting stromal glutamine synthetase in tumors disrupts tumor microenvironment-regulated cancer cell growth. Cell Metab. 2016;24:685–700. doi:10.1016/j.cmet.2016.10.011.
- Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, Tai Y, Tang Y, Zhang YY, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest. 2016;126:3313–3335. doi:10.1172/JCI86387.
- Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, Hale A, Bhat B, Kaimal V, Zhang YY, et al. Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ-miR-130/301 circuit. Cell Rep. 2015;13:1016–1032. doi:10.1016/j.celrep.2015.09.049.
- Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, Chen J. AMPK modulates hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17:490–499. doi:10.1038/ncb3137.
