ABSTRACT
Basal-like breast cancers have the highest rates of locoregional recurrence after radiation. By correlating gene expression with early locoregional recurrence, we nominate TTK protein kinase as a mediator of radioresistance. TTK inhibition radiosensitizes in vitro and in vivo through a novel mechanism of impaired homologous recombination and represents a promising translational strategy.
Outcomes for breast cancer patients have improved significantly over the past several decades; however, basal-like breast cancers, have significantly worse outcomes than other breast cancer subtypes.Citation1 While targeted therapies are available for estrogen receptor-positive (ER+) and human epidermal growth factor receptor 2-positive (Her2+) breast cancers, few effective therapies are available for patients with basal-like breast cancer. Radiation therapy (RT) remains a mainstay of current clinical management of breast cancer but is least effective in women with basal-like breast cancer, though the molecular drivers of this radioresistance are currently unknown.Citation2,Citation3 Given the fundamental lack of knowledge regarding the mediators of radiation resistance and a furthered lack of targeted agents for basal-like and triple-negative breast cancer (TNBC), it is clear that the development of additional targets for radiosensitization represents a critical unmet clinical need.
To address this gap in understanding and in an effort to identify mediators of locoregional recurrence in breast cancer patients, we hypothesized that the mediators of radiation resistance were intrinsic to the tumors themselves. To that end, we correlated primary tumor gene expression with early local recurrence (<3 years) across four independent clinical breast cancer datasets in which women received adjuvant radiation therapy as part of their care.Citation4 Although numerous genes were associated with early local recurrence in each individual dataset, only 10 genes were significantly correlated with local recurrence across all four datasets. The top nominated gene by differential expression, TTK protein kinase (TTK) (also known as Monopolar spindle 1 [MPS1]), is significantly elevated in breast cancer compared to normal tissue. In addition, TTK expression is significantly higher in basal-like breast cancer compared to other subtypes in both The Cancer Genome Atlas (TCGA) and Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) datasets.
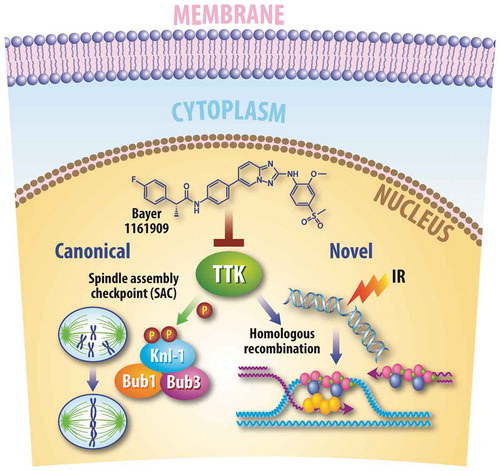
Canonically, TTK is an essential cell cycle-regulating kinase at the apex of the spindle assembly checkpoint (SAC) complex.Citation5 This checkpoint, between metaphase and anaphase of mitosis, is responsible for the accurate alignment of chromosomes during metaphase.Citation6 To initiate the checkpoint, TTK phosphorylates kinetochore scaffold 1 (KNL1) leading to the recruitment of additional SAC proteins and delaying the transition to anaphase.Citation7 Inhibition of TTK has been shown to increase mitotic errors including mitotic missegregation, anaphase bridges, and aneuploidy across multiple cancer models, including TNBC.Citation8,Citation9
Using both genetic (shRNA/siRNA) and pharmacologic (clinical grade ATP-competitive inhibitor of TTK) inhibition of TTK, we show significant radiosensitization in multiple basal-like breast cancer cell lines. Reintroduction of wild-type TTK, after knockdown of endogenous TTK, rescued radioresistance; however, the reintroduction of kinase-dead mutant TTK was unable to confer this radioresistance, indicating inhibition of TTK’s kinase function renders basal-like breast cancer cell lines more sensitive to RT.
Combination treatment of TTK inhibition (both genetic and pharmacologic) and RT led to persistent unresolved double-strand DNA (dsDNA) breaks over time, as measured by γH2AX foci, in multiple basal-like breast cancer cell lines, indicating radiosensitization was mediated, at least in part, through impaired dsDNA break repair. Using a specific homologous recombination (HR) reporter system, we show TTK inhibition significantly decreases HR efficiency. Furthermore, TTK knockdown in combination with RT significantly disrupts RAD51 recombinase (RAD51) foci formation, a marker for active HR.Citation10 Reintroduction of wild-type TTK after endogenous TTK knockdown rescues HR repair efficiency as well as RAD51foci formation, while the reintroduction of kinase-dead mutant TTK did not rescue this HR repair efficiency. TTK inhibition had no effect on the other major dsDNA break repair pathway, non-homologous end joining (NHEJ). Together, our results demonstrate that TTK inhibition leads to increased unresolved dsDNA breaks over time that is due, at least in part, to reduced the efficiency of the HR repair pathway. This is one of the first studies to demonstrate a cell-cycle and SAC-independent role for TTK and a novel role in HR repair efficiency.
In vivo, we show TTK knockdown in combination with RT significantly delays tumor growth and increases time to tumor tripling compared to either TTK inhibition or RT alone. TTK inhibition, using a clinical grade inhibitor, in combination with RT, synergistically delayed tumor growth compared to either TTK inhibition or RT alone. Finally, TTK inhibition in combination with RT synergistically decreased tumor growth in an orthotopic patient-derived xenograft (PDX) model compared to either drug or RT alone. In general, TTK inhibition was well tolerated in mice as evidence by their lack of discernable difference in appearance, activity, or weight.
Taken together, our results suggest a clinically feasible strategy to radiosensitize basal-like breast cancer by inhibiting TTK function during radiation treatment. While we show TTK inhibition reduces HR efficiency, previous groups have established that inhibition of TTK increases mitotic instability and chromosomal abnormalities. This suggests that inhibition of TTK likely leads to radiosensitization through multiple mechanisms, potentially increasing the efficacy of treatment. Our results add to previous literature showing TTK inhibition may be a feasible clinical strategy for patients and suggest improved tolerability of this approach as TTK inhibition-mediated radiosensitization occurs at very low concentrations of the drug, far below levels that produced toxicity in previous phase I trials of TTK inhibitors. These results also suggest a novel, non-canonical role for TTK function in mediating dsDNA break repair through interaction with the HR pathway that impacts radiosensitivity in aggressive basal-like models of breast cancer ().
Figure 1. Genomic stabilization by TTK. Inhibition of TTK protein kinase (TTK) leads to an increase in aneuploidy, lagging chromosomes, and mitotic catastrophe through its canonical role in the spindle assembly checkpoint (SAC) complex. Our findings demonstrate a novel role for TTK in the homologous recombination (HR) pathway. Inhibition of TTK with knockdown, kinase-dead constructs, and a clinical grade inhibitor, Bayer 1161909, decreases in the efficiency of HR but has no effect on non-homologous end joining (NHEJ). IR – ionizing radiation.
Disclosure of potential conflicts of interest
The authors have declared that no conflicts of interest exist.
Additional information
Funding
References
- Atlanta ACS. Cancer facts & figures 2020. Am Can Soc. 2020;1:12.
- Kyndi M, Sorensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26(9):1–3. doi:10.1200/JCO.2007.14.5565.
- Lowery AJ, Kell MR, Glynn RW, Kerin MJ, Sweeney KJ. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat. 2012;133(3):831–841. doi:10.1007/s10549-011-1891-6.
- Chandler BC, Moubadder L, Ritter CL, Liu M, Cameron M, Wilder-Romans K, Zhang A, Pesch AM, Michmerhuizen AR, Hirsh N, et al. TTK inhibition radiosensitizes basal-like breast cancer through impaired homologous recombination. J Clin Invest. 2020;130(2):958–973. doi:10.1172/JCI130435.
- Poch O, Schwob E, de Fraipont F, Camasses A, Bordonné R, Martin RP. RPK1, an essential yeast protein kinase involved in the regulation of the onset of mitosis, shows homology to mammalian dual-specificity kinases. Mol Gen Genet MGG. 1994;243(6):641–653. doi:10.1007/BF00279573.
- Musacchio A, Salmon ED, Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8(5):379–393. doi:10.1038/nrm2163.
- Faesen AC, Thanasoula M, Maffini S, Breit C, Müller F, van Gerwen S, Bange T, Musacchio A. Basis of catalytic assembly of the mitotic checkpoint complex. Nature. 2017;542(7642):498–502. doi:10.1038/nature21384.
- Mason JM, Wei X, Fletcher GC, Kiarash R, Brokx R, Hodgson R, Beletskaya I, Bray MR, Mak TW. Functional characterization of CFI-402257, a potent and selective Mps1/TTK kinase inhibitor, for the treatment of cancer. Proc National Acad Sci. 2017;114(12):3127. doi:10.1073/pnas.1700234114.
- Thu KL, Silvester J, Elliott MJ, Ba-alawi W, Duncan MH, Elia AC, Mer AS, Smirnov P, Safikhani Z, Haibe-Kains B, et al. Disruption of the anaphase-promoting complex confers resistance to TTK inhibitors in triple-negative breast cancer. Proc National Acad Sci. 2018;115(7):E1570. doi:10.1073/pnas.1719577115.
- Cruz C, Castroviejo-Bermejo M, Gutiérrez-Enríquez S, Llop-Guevara A, Ibrahim YH, Gris-Oliver A, Bonache S, Morancho B, Bruna A, Rueda OM, et al. RAD51 foci as a functional biomarker of homologous recombination repair and PARP inhibitor resistance in germline BRCA-mutated breast cancer. Ann Oncol. 2018;29(5):1203–1210. doi:10.1093/annonc/mdy099.
