ABSTRACT
Centrosomes are not absolutely essential for cell division; acentrosomal bipolar spindles can be established in oocytes and centrosome-eliminated somatic cells. However, the detailed mechanisms describing how spindle bipolarity is established without centrosomes are not completely understood. We have recently demonstrated that in acentrosomal human cells, nuclear mitotic apparatus protein (NuMA) assemblies-mediated microtubule asters and EG5 promote spindle bipolarization in early mitosis.
The centrosome consists of one or two centrioles, which are surrounded by pericentriolar material.Citation1 Centrosomes serve as microtubule-organizing centers (MTOCs) in both interphase and mitosis. Centrosomes nucleate and organize microtubules and are separated from each other through the actions of centrosome separation machinery at the G2/M transition ().Citation2 Timely separation of the centrosomes is crucial for efficient bipolar spindle assembly and subsequent proper chromosome segregation in mitosis.
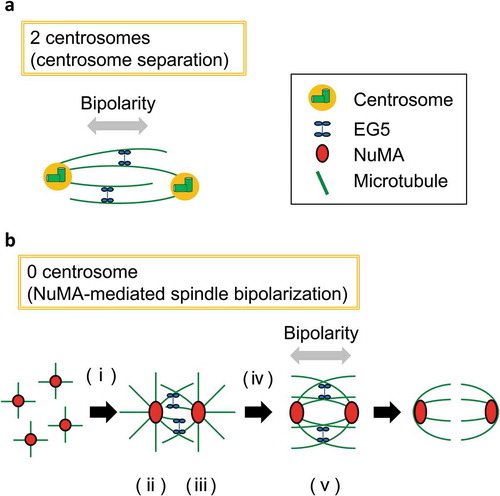
Figure 1. Models for centrosomal and acentrosomal pathways that promote spindle bipolarization in human cells.
A. Centrosomes serve as microtubule organizing centers in somatic cells. EG5 crosslinks and slides anti-parallel microtubules which nucleated from centrosomes to promote centrosome separation at G2/M transition. This centrosome separation pathway is crucial for bipolar spindle assembly B. The model of how nuclear mitotic apparatus protein (NuMA) promotes spindle bipolarization in acentrosomal human cells suggested in our recent study. (i) NuMA assemblies organize microtubule asters. (ii) The clustering activity of NuMA and dynein promote the assembly of NuMA to support the formation of a radial array of microtubules. (iii) The radial array of microtubules incorporates EG5. (iv) EG5 may be activated and/or loaded at the appropriate position on cross-linked microtubules. (v) The motor activity of EG5 on cross-linked microtubules promotes separation of the spindle poles.
Of note, in most animal species, including humans, oocytes do not have centrosomes. Instead of centrosomes, acentrosomal meiotic machineries promote bipolar spindle formation in oocytes. In mouse oocytes, MTOCs containing pericentriolar material components, such as pericentrin and γ-tubulin, are formed de novo from a cytoplasmic microtubule network during prophase.Citation3 Multiple MTOCs nucleate microtubules cooperatively with Ran-GTP to form a microtubule ball with several γ-tubulin-positive foci on the surface.Citation3 These foci eventually cluster in an EG5-dependent manner, enabling the cluster to achieve a bipolar state.Citation3 In human oocytes, it has been shown that the Ran-GTP-dependent microtubule nucleation pathway is important for spindle assembly.Citation4 In addition to oocytes, vertebrate somatic cells can divide in the absence of centrosomes under certain conditions. However, the detailed mechanisms of spindle bipolarization in the acentrosomal spindle are not thoroughly understood.
Our recent research has shown that, in acentrosomal human cells, aggregates of nuclear mitotic apparatus protein (NuMA) organize microtubule asters to establish spindle bipolarity in early mitosis.Citation5 NuMA is a factor involved in spindle pole organization and spindle positioning in mitosis. It interacts with microtubules,Citation6,Citation7 and organizes microtubule asters independently of centrosomes.Citation6–Citation8 Although the properties of NuMA for organizing microtubule asters appear to be similar to those of centrosomes in early mitosis, its function in spindle bipolarization of acentrosomal human cells remains poorly understood.
In a recent study, we induced an acentrosomal spindle through inhibition of Polo Like Kinase 4 (PLK4) using centrinone, a PLK4-specific inhibitor,Citation9 to examine this function of NuMA. We carefully observed the assembly processes of mitotic spindles, which lacked centrioles. The results showed that NuMA assembled small asters of microtubules during or after nuclear envelope breakdown. Multiple NuMA-mediated microtubule asters were assembled into one or two radial arrays of microtubules and incorporated EG5, a critical kinesin motor involved in spindle bipolarization.Citation10 These acentrosomal cells subsequently established a small bipolar spindle.
We then analyzed the mechanisms of: (1) the assembly of NuMA and (2) spindle bipolarization in acentrosomal human cells. NuMA is transported toward the spindle poles in a dynein motor-dependent manner and possesses clustering activity.Citation11–Citation14 Therefore, we examined whether these mechanisms are also implicated in the assembly of NuMA and subsequent acentrosomal spindle formation. We found that depletion of the dynein heavy chain 1 by the Auxin-inducible-degron system suppressed the assembly of NuMA in acentrosomal human cells.Citation5,Citation15 Furthermore, the replacement of endogenous NuMA with mutants which lack clustering activity inhibited the assembly of NuMA in acentrosomal spindle formation. These results suggest that, in the absence of centrosomes, the assembly of NuMA is supported by dynein and its own intrinsic clustering properties.
We further investigated the exact function of NuMA in spindle bipolarization in acentrosomal cells. Using the Auxin-inducible-degron system,Citation14 our analysis showed that upon NuMA depletion, acentrosomal cells failed to organize the radial array of microtubules that normally assembles before bipolar spindle formation. Instead, NuMA-depleted cells assembled an umbrella-like abnormal monopolar spindle. EG5 was recruited onto the microtubules around NuMA assemblies and was crucial for establishing spindle bipolarization in acentrosomal cells. Therefore, it is conceivable that the NuMA assemblies organize cross-linked microtubules, which incorporate EG5 for spindle pole separation. These recent results suggest that, at the initial step of spindle bipolarization in acentrosomal cells, NuMA assemblies organize microtubules in a radial array. Subsequently, EG5 is recruited at the appropriate position on these microtubules to promote spindle pole separation.
Considering these results, we propose the following model ():
At the time of nuclear envelope breakdown, NuMA assemblies organize microtubule asters.
The clustering activity of NuMA and dynein promote the assembly of NuMA to support the formation of a radial array of microtubules.
The radial array of microtubules incorporates EG5.
EG5 may be activated and/or loaded at the appropriate position on cross-linked microtubules.
The motor activity of EG5 on cross-linked microtubules promotes separation of the spindle poles.
In addition, it is important to determine whether the mechanisms identified in this study are also involved in meiotic spindle formation in human oocytes. The characteristic feature of meiotic spindle assembly in human oocytes is that chromosomes and Ran-GTP are used for the initial assembly of spindle microtubules. However, the detailed mechanisms leading to the establishment of bipolarity in these spindles remain to be clarified. It has been shown previously that NuMA accumulates in the center of monopolar-like spindles in pre-meiosis I in human oocytes.Citation16 The structure of NuMA observed was similar to the NuMA assemblies observed in this study, raising the possibility that NuMA-mediated establishment of spindle bipolarity also occurs in human oocyte meiosis. The present study may provide insights into the systems regulating meiotic spindle assembly in human oocytes, and future studies are warranted to test this hypothesis.
Acknowledgments
We are grateful to all the coauthors of our original paper for completing the study. We also thank the people who gifted us the cell lines and reagents used in the study.
Disclosure Statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Nigg EA, Holland AJ. Once and only once: mechanisms of centriole duplication and their deregulation in diseases. Nat Rev Mol Cell Biol. 2018;19(5):1–3. doi:10.1038/nrm.2017.127.
- Agircan FG, Schiebel E, Mardin BR. Separate to operate: control of centrosome positioning and separation. Philos Trans. R Soc B Biol Sci. 2014;369(1650):20130461. doi:10.1098/rstb.2013.0461.
- Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell. 2007;130(3):484–498. doi:10.1016/j.cell.2007.06.025.
- Holubcová Z, Blayney M, Elder K, Schuh M. Error-prone chromosome-mediated spindle assembly favors chromosome segregation defects in human oocytes. Science. 2015;348(6239):1143–1147. doi:10.1126/science.aaa9529.
- Chinen T, Yamamoto S, Takeda Y, Watanabe K, Kuroki K, Hashimoto K, Takao D, Kitagawa D. NuMA assemblies organize microtubule asters to establish spindle bipolarity in acentrosomal human cells. Embo J. 2020;39(2):e102378. doi:10.15252/embj.2019102378.
- Haren L, Merdes A. Direct binding of NuMA to tubulin is mediated by a novel sequence motif in the tail domain that bundles and stabilizes microtubules. J Cell Sci. 2002;115:1815–1824.
- Du Q, Taylor L, Compton DA, Macara IG. LGN blocks the ability of NuMA to bind and stabilize microtubules. A mechanism for mitotic spindle assembly regulation. Curr Biol. 2002;12(22):1928–1933. doi:10.1016/S0960-9822(02)01298-8.
- Gaglio T, Saredi A, Compton DA. NuMA is required for the organization of microtubules into aster-like mitotic arrays. J Cell Biol. 1995;131(3):693–708. doi:10.1083/jcb.131.3.693.
- Wong YL, Anzola JV, Davis RL, Yoon M, Motamedi A, Kroll A, Seo CP, Hsia JE, Kim SK, Mitchell JW, et al. Reversible centriole depletion with an inhibitor of Polo-like kinase 4. Science. 2015;348(6239):1155–1160. doi:10.1126/science.aaa5111.
- Mann BJ, Wadsworth P. Kinesin-5 regulation and function in mitosis. Trends Cell Biol. 2019;29(1):66–79. doi:10.1016/j.tcb.2018.08.004.
- Merdes A, Ramyar K, Vechio JD, Cleveland DW. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87(3):447–458. doi:10.1016/S0092-8674(00)81365-3.
- Hueschen CL, Kenny SJ, Xu K, Dumont S. NuMA recruits dynein activity to microtubule minus-ends at mitosis. Elife. 2017;6:e29328. doi:10.7554/eLife.29328.
- Merdes A, Heald R, Samejima K, Earnshaw WC, Cleveland DW. Formation of spindle poles by dynein/dynactin-dependent transport of numa. J Cell Biol. 2000;149(4):851–862. doi:10.1083/jcb.149.4.851.
- Okumura M, Natsume T, Kanemaki MT, Kiyomitsu T. Dynein–Dynactin–NuMA clusters generate cortical spindle-pulling forces as a multi-arm ensemble. Elife. 2018;7:e36559. doi:10.7554/eLife.36559.
- Natsume T, Kiyomitsu T, Saga Y, Kanemaki MT. Rapid protein depletion in human cells by auxin-inducible degron tagging with short homology donors. Cell Rep. 2016;15(1):210–218. doi:10.1016/j.celrep.2016.03.001.
- Xu X, Duan X, Lu C, Lin G, Lu G. Dynamic distribution of NuMA and microtubules in human fetal fibroblasts, developing oocytes and somatic cell nuclear transferred embryos. Hum Reprod. 2011;26(5):1052–1060. doi:10.1093/humrep/der067.
