ABSTRACT
Disruption of chromatin structure could enable early carcinogenesis by facilitating malignant transformation. Using stochastic optical reconstruction microscopy optimized for pathological tissue (PathSTORM), we uncovered a gradual decompaction of higher-order chromatin folding through progressive stages of carcinogenesis. We demonstrated potential detection of pre-cancerous genomic architecture not easily discernible by conventional pathology.
Compaction of genomic DNA into a higher-order chromatin structure regulates gene expression by facilitating or inhibiting transcription. The fundamental unit of chromatin, the nucleosome, is organized into regions of open, transcriptionally active euchromatin and closed, transcriptionally repressed heterochromatin filled with repetitive elements that maintain genome stability.Citation1 Decompaction of constitutive heterochromatic structural regions, defined by the presence of histone H3 lysine 9 trimethylation (H3K9me3), is a hallmark characteristic of many cancer cells and is often used in clinical diagnosisCitation2 because the resulting genomic instability allows cells to exhibit permissive or “plastic” characteristics. These permissive states may allow stochastic oncogene activation, resulting in non-physiologic cell fate transitions, with some changes driving tumorigenesis and the acquisition of malignancyCitation3 as well as morphological changes to cells and their nuclei discernible using conventional microscopy.Citation2
Figure 1. Advances enabled by PathSTORM in the fields of microscopy, clinical diagnosis, and academic research. (a) Other STORM methods have broken the diffraction barrier limiting conventional light microscopy to a resolution of ~250 nm, and PathSTORM compares to other optimized STORM protocols, resulting in a resolution of ~30 nm. While other STORM technologies are only able to image thin, homogenous samples, PathSTORM and light microscopy can image both these and complex, pathological tissue samples. (b) CUT&RUN enables the acquisition of high-quality, low-background protein localization on chromatin, and found decreased H3K9me3 occupancy and increased H3K4me3. RNA-seq data confirmed that these architectural changes influenced transcription at differentially organized locations, and Gene Ontology (GO)-term analysis identified that the most significantly enriched pathways were mesenchymal development (a process leading to acquisition of invasiveness and malignancy in epithelial cancer cells), cellular response to DNA damage stimulus, and mitotic cell cycle process critical for maintaining genomic stability. (c) PathSTORM improves upon conventional cancer diagnostic methods (such as H&E staining) by enabling the potential to characterize the genomic architecture preceding the onset of tumorigenesis, where conventional methods rely on visualizing the morphological changes of the cell and cell nucleus after the onset of dysregulation.
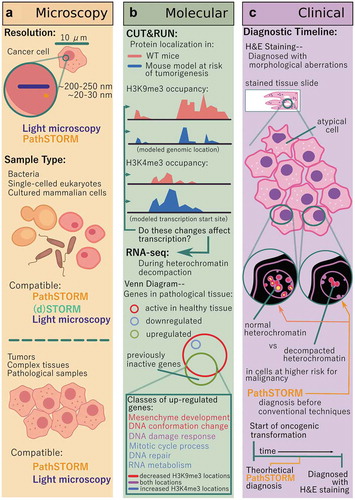
While aberrations to nuclear size and shape in cancer cells have been observable by light microscopy for a century and a half, the molecular-scale structure of dysfunctional heterochromatin has remained unknown, since traditional analyses toward determining the mechanisms leading to these phenotypic changes have been performed using biochemical interrogation of DNA sequences and chromatin-bound proteins. Visualizing higher-order chromatin structure requires a method that can obtain image information below the resolution limit of a conventional light microscope. Advances in super-resolution fluorescence microscopy have overcome the diffraction barrier that limits the resolution of a conventional microscope to ~200-250 nm, corresponding to about half of the smallest visible light wavelength.Citation4 Stochastic optical reconstruction microscopy (STORM) has been used to provide imaging data down to ~20-30 nm, the scale of nucleosome clusters,Citation5 but this technique had mostly been optimized for use on simple model systems like bacteria or thin, cultured cells. These highly transparent systems with uniform image background have been necessary for accurate localization of individual molecules. Imaging pathological tissue has been much more difficult as greater auto-fluorescence and stronger scattering results in higher background signal.
Finding a way to optimize STORM imaging for pathological tissue remained a tantalizing goal. If chromatin from cancerous and pre-cancerous tissues could be imaged in high resolution, answers to a number of questions could then be elucidated: Is there a specific, visible chromatin abnormality in cancer cells, and how early is it discernible? What is the chromatin landscape in early carcinogenesis, before visible tumor formation? How does chromatin architecture change throughout cancer progression? Will these changes in chromatin landscape enable cancer diagnosis and prognosis? Utilizing super-resolution imaging methods for clinical diagnoses would require a technique that allows for high-throughput processing using formalin-fixed, paraffin-embedded tissue. Thus, we saw a need for a technique that met these requirements for use in a clinical context and that addressed previous technical shortcomings for imaging pathological tissues.
Enter PathSTORM – a super-resolution technique optimized for the robust, reproducible, and high-quality imaging of heterogeneous pathological tissues ().Citation6 This technique incorporated three major advances in STORM imaging: reduction in background signal through optical clearing and buffer optimization, an enhanced background correction algorithm, and a high-speed, high-fidelity image reconstruction algorithm. With these optimizations, reproducible high-quality super-resolution imaging of pathological samples suddenly became possible. Excited at the potential to visualize in situ pathological tissues in a clinical setting, we visualized the initiation and stepwise progression of murine precancerous and cancerous heterochromatin to compare to that from normal, non-anomalous tissues.Citation6 What we found was a decompaction of mouse heterochromatin over the course of tumorigenesis before morphological changes to cells visible using conventional microscopy. This decompaction was a gradual process, not one sparked by a singular event and completed quickly. Imaging human neoplasia revealed results consistent with these mouse models.
Complementing our imaging analysis with state-of-the-art molecular techniques provided information about the genomic localization of chromatin-structure aberrations and resulting gene expression changes. Using a genome-scale protein localization technique called CUT&RUN (an alternative to chromatin immunoprecipitationCitation7) used for its decreased background signal and low cell/chromatin input requirementsCitation8 we saw a reduction in H3K9me3 occupancy at heterochromatic genomic locations in tissues at-risk for tumorigenesis while total H3 levels remained constant, suggesting more open chromatin genome-wide ().Citation6 Subsequent RNA-seq analysis demonstrated increased expression in genes associated with increased risk for malignancy in epithelial cells. An increase in markers for DNA double-stranded breaks implied increases in genomic stability. These results supported our imaging of disrupted heterochromatin structure identified by PathSTORM and provided molecular details about the state of cell genomes in early carcinogenesis.
We anticipate PathSTORM can be applied broadly, and the success of our research has motivated us to consider clinical applications. If further experimentation confirmed that the differences in chromatin architecture visible using PathSTORM can identify truly aggressive pre-cancerous lesions and stratify patients at highest risk for developing cancer in humans, visualizing pre-cancerous genome folding and stability changes can improve clinical diagnosis and early cancer detection. The specific abnormalities we observed seem to be characteristic of cancer cells, and their gradual disruption of chromatin compaction seems to correlate with all stages of carcinogenesis. Moreover, because PathSTORM takes a snapshot of cells, pinpointing the timeline along the process of carcinogenesis and prognosis (or risk stratification) might become possible. While conventional histology (H&E staining) remains the gold standard as the first-line diagnosis,Citation9 PathSTORM may prove to be a superior tool in cases difficult for pathologists to identify. The level of available image detail and sensitivity far out-shine the technical capabilities of conventional light microscopy and robust architectural changes in chromatin might overcome the inherent molecular heterogeneity that limits the performance of other biomarker analyses. Furthermore, given the availability of cost-effective STORM imaging systems,Citation10 the cost for PathSTORM imaging of patient samples could be comparable to the commonly used pathological assays such as immunohistochemistry (IHC).
Additional work is still required to utilize PathSTORM to its full potential in early cancer detection. Importantly, super-resolution microscopy has not been applied in a clinical setting. Standardization of sample preparation and rigorous quality control need to be established for highly reproducible super-resolution images of chromatin architecture in clinical samples. Further mechanistic understanding of the functional roles of chromatin architecture in carcinogenesis is also needed. However, PathSTORM has the potential to fundamentally improve the way we diagnose cancer or risk-stratify precursors – ultimately, a path worth exploring.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Even-Faitelson L, Hassan-Zadeh V, Baghestani Z, Bazett-Jones DP. Coming to terms with chromatin structure. Chromasoma. 2016;125:1–3. doi:10.1007/s00412-015-0534-9.
- Morgan MA, Shilatifard A. Chromatin signatures of cancer. Genes Dev. 2015;29:238–249. doi:10.1101/gad.255182.114.
- Flavahan WA, Gaskell E, Bernstein BE. Epigenetic plasticity and the hallmarks of cancer. Science. 2017;357:266. doi:10.1126/science.aal2380.
- Sigal YM, Zhour R, Zhuang X. Visualizing and discovering cellular structures with super-resolution microscopy. Science. 2018;361:880–887. doi:10.1126/science.aau1044.
- Xu J, Ma H, Jin J, Uttam S, Fu R, Huang Y, Liu Y. Super-resolution imaging of higher-order chromatin at different epigenetic states in single mammalian cells. Cell Rep. 2018;24(4):873–882. doi:10.1016/j.celrep.2018.06.085.
- Xu J, Ma H, Ma H, Jiang W, Mela CA, Duan M, Zhao S, Gao C, Hahm E, Lardo SM, et al. Super-resolution imaging reveals the evolution of higher-order chromatin folding in early carcinogenesis. Nat Com. 2020;11:1899. doi:10.1038/s41467-020-15718-7.
- Skene PJ, Henikoff S. An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. eLife. 2017;6:e21856. doi:10.7554/eLife.21856.
- Hainer SJ, Boskovic A, McCannell KN, Rando OJ, Fazzio TG. Profiling of pluripotency factors in single cells and early embryos. Cell. 2019;177:1319–1329. doi:10.1016/j.cell.2019.03.014.
- Chen JM, Li Y, Xu J, Gong L, Wang LW, Liu WL, Liu J. Computer-aided prognosis on breast cancer with hematoxylin and eosin histopathology images: A review. Tumor Biol. 2017;39(3):101042831769455. doi:10.1177/1010428317694550.
- Ma H, Fu R, Xu J, Liu Y. A simple and cost-effective setup for super-resolution localization microscopy. Sci Rep. 2017;7:1542. doi:10.1038/s41598-017-01606-6.
