ABSTRACT
Mixed lineage kinase domain-like protein (MLKL) is the proposed executioner of necroptosis. Our recent findings identify a novel inhibitor necroptosis-blocking compound 1 (NBC1) which specifically conjugates to two cysteines of heat shock protein 70 (HSP70) to block its function. Importantly, HSP70 promotes MLKL polymerization to activate necroptosis.
Author’s view
The lytic cell death pathway, necroptosis, has emerged as a critically important mode of regulated cell death over the past two decades.Citation1 Phenotypically, necroptosis is indistinguishable from necrosis, identified by organelle swelling, loss of plasma membrane integrity, and release of cytoplasmic contents including pro-inflammatory mediators or damage associated molecular patterns (DAMPs), which magnify the innate inflammatory response. Necroptosis also stimulates a cell-autonomous increase in cytokine production that contributes to the inflammatory milieu and paracellular signaling. The pro-inflammatory nature of necroptosis is double-edged, as it contributes to sustained cellular injury as well as protective stimulation of the adaptive immune system. This dichotomy is exemplified in cancer, where necroptosis has both anti- and pro-tumor effects.Citation2
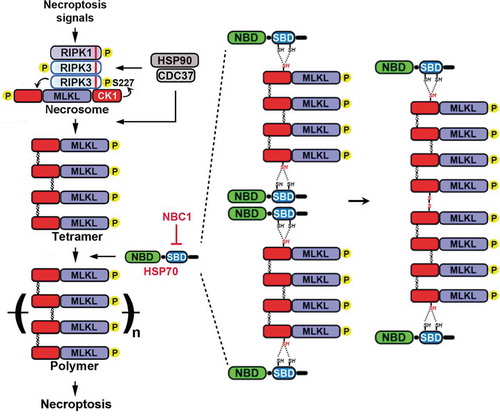
The canonical necroptosis pathway relies on a multimillion Dalton complex called the necrosome, which includes two kinases, receptor interacting protein kinase 1 and 3 (RIPK1, RIPK3), and a pseudokinase, mixed lineage kinase domain-like protein (MLKL) ().Citation1 Upstream signal promotes RIPK1 interaction with RIPK3 through RIP homotypic interaction motif (RHIM), leading to RIPK3 auto-phosphorylation and homo-oligomerization. RIPK3 homo-oligomer then recruits the casein kinase 1 (CK1) family proteins, CK1α, CK1δ and CK1ε, which phosphorylate serine 227 of human RIPK3, enabling RIPK3 to recruit and phosphorylate MLKL.Citation3 Phosphorylated MLKL undergoes a conformational change, leading to formation of MLKL tetramers which subsequent polymerize into disulfide bond-dependent, amyloid-like fibers.Citation4 Through mechanisms that remain unclear, the MLKL polymers contribute to plasma membrane rupture and cell death. We wanted to determine if additional protein mediators contributed to the steps of terminal necroptosis.
Figure 1. Molecular mechanisms of necroptosis. Upstream necroptosis signals activate the assembly of the necrosome, which contains receptor-interacting protein kinase 1 and 3 (RIPK1, RIPK3) and mixed lineage kinase-like protein (MLKL), as well as casein kinase 1 family proteins α, δ and ε (CK1). Phosphorylation of RIPK3 at serine 227 by CK1 enables RIPK3 to recruit and phosphorylate MLKL. With the help of heat shock protein 90 (HSP90) and its co-chaperone cell division cycle 37 (CDC37), phosphorylated MLKL forms tetramers. Subsequently, heat shock protein 70 (HSP70) uses two cysteines in its substrate binding domain (SBD) to promote polymerization of MLKL tetramers and necroptosis execution. Novel inhibitor necroptosis-blocking compound 1 (NBC1) conjugates these two cysteines of HSP70 to block MLKL polymerization. NBD, nucleotide binding domain. Please see text for details.
Using an unbiased, forward small molecule screen, we identified a novel necroptosis inhibitor named necroptosis blocking compound 1 (NBC1).Citation5 NBC1 contains three putative cysteine-targeting Michael acceptors, and at least two of which were required to efficiently block necroptosis. NBC1 did not inhibit phosphorylation of RIPK1, RIPK3, or MLKL, nor did it inhibit MLKL tetramer formation. However, it inhibited MLKL polymerization, suggesting that MLKL tetramers are not sufficient for cell death induction, instead MLKL polymers are required. Furthermore, NBC1 also inhibited necroptosis induced by the polymerization of the N-terminal domain (NTD) of MLKL.
Biotinylated NBC1, but not a NBC1 derivative with only one Michael acceptor, specifically conjugated to heat shock protein 70 (HSP70, HSPA1A). HSP70 is a stress inducible heat shock protein that regulates protein stability and folding through an N-terminal nucleotide-binding domain (NBD) and a C-terminal substrate binding domain (SBD).Citation6 In an in vitro assay, HSP70 promoted polymerization of the NTD of MLKL in an ATP-independent manner. Furthermore, HSP70-SBD alone was sufficiently to promote MLKL-NTD polymerization, and NBC1 specifically conjugated cysteine 574 and cysteine 603 of the SBD to block MLKL-NTD polymerization. These results prompted us to propose the following model (). Phosphorylation of MLKL induces its conformational changes to form disulfide bond-dependent tetramers. HSP70 interacts with exposed short hydrophobic peptides in the newly formed tetramer, and uses cysteines 574 and 603 to protect and maybe activate other cysteines in the tetramer. Binding of HSP70 with the tetramer could also shield the tetramer from the reducing power of thioredoxin 1, which we have previously shown directly interacts with MLKL and keeps MLKL in a reduced state.Citation7 HSP70-associated MLKL tetramer is then delivered to the growing MLKL polymer and forms proper disulfide bonds with the polymer. Once the tetramer incorporates into the polymer, it dissociates from HSP70 possibly because of steric hindrance, and the released HSP70 will start a new cycle again. This also explains why ATP is not needed for HSP70 substrate dissociation.
In addition, HSP70 works in concert with heat shock protein 90 (HSP90) and its co-chaperone cell division cycle 37 (CDC37) during necroptosis (). The HSP90/CDC37 complex associates with RIPK3 to facilitate its phosphorylation of MLKL, followed by its association with MLKL to facilitate MLKL tetramer formation.Citation8,Citation9 Subsequently, HSP70 associates with the NTD of MLKL tetramers and promotes MLKL polymerization.
Previous publications have shown a divergent function of HSP70 in necroptosis.Citation10 Inhibition of HSP70’s nucleotide binding domain with the allosteric inhibitor JG-98 and analogs destabilizes RIPK1 regulators, causing apoptosis and, with caspase inhibition, necroptosis. Yet, results were not consistent across all transformed cell lines. Thus, the consequence of HSP70 inhibition in cancer cells is tumor type-dependent and compound-dependent, emphasizing the complex and variable cell signaling landscape in cancer. Heat shock proteins are integral to de novo protein folding, prevention of aggregation, and refolding misfolded proteins. These features promote cellular viability and thus are essential to tumor development and metastasis.Citation6 Although the anti-tumor effects of necroptosis may have therapeutic promise, increased MLKL expression and the inflammatory microenvironment caused by necroptosis promote metastasis.Citation2 Temporal-spatial therapeutic targeting of both HSP70 and necroptosis to limit metastasis bears further exploration.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Galluzzi L, Kepp O, Chan FK, Necroptosis: KG. Mechanisms and relevance to disease. Annu Rev Pathol. 2017;12:1–3. doi:10.1146/annurev-pathol-052016-100247.
- Zhu F, Zhang W, Yang T, He SD. Complex roles of necroptosis in cancer. J Zhejiang Univ Sci B. 2019;20:399–413. doi:10.1631/jzus.B1900160.
- Hanna-Addams S, Liu S, Liu H, Chen S, Wang Z. CK1alpha, CK1delta, and CK1epsilon are necrosome components which phosphorylate serine 227 of human RIPK3 to activate necroptosis. Proc Natl Acad Sci U S A. 2020;117:1962–1970. doi:10.1073/pnas.1917112117.
- Liu S, Liu H, Johnston A, Hanna-Addams S, Reynoso E, Xiang Y, Wang Z. MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis. Proc Natl Acad Sci U S A. 2017;114:E7450–E7459. doi:10.1073/pnas.1707531114.
- Johnston AN, Ma Y, Liu H, Liu S, Hanna-Addams S, Chen S, Chen C, Wang Z. Necroptosis-blocking compound NBC1 targets heat shock protein 70 to inhibit MLKL polymerization and necroptosis. Proc Natl Acad Sci U S A. 2020;117:6521–6530. doi:10.1073/pnas.1916503117.
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi:10.1007/s00018-004-4464-6.
- Reynoso E, Liu H, Li L, Yuan AL, Chen S, Wang Z. Thioredoxin-1 actively maintains the pseudokinase MLKL in a reduced state to suppress disulfide bond-dependent MLKL polymer formation and necroptosis. J Biol Chem. 2017;292:17514–17524. doi:10.1074/jbc.M117.799353.
- Li D, Xu T, Cao Y, Wang H, Li L, Chen S, Wang X, Shen Z. A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc Natl Acad Sci U S A. 2015;112:5017–5022. doi:10.1073/pnas.1505244112.
- Jacobsen AV, Lowes KN, Tanzer MC, Lucet IS, Hildebrand JM, Petrie EJ, van Delft MF, Liu Z, Conos SA, Zhang J-G, et al. HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis. 2016;7:e2051. doi:10.1038/cddis.2015.386.
- Srinivasan SR, Cesa LC, Li X, Julien O, Zhuang M, Shao H, Chung J, Maillard I, Wells JA, Duckett CS, et al. Heat shock protein 70 (Hsp70) suppresses RIP1-dependent apoptotic and necroptotic cascades. Mol Cancer Res. 2018;16:58–68. doi:10.1158/1541-7786.MCR-17-0408.
