ABSTRACT
Cancer stem cells (CSCs) underlie resistance to therapy. Cancer develops only in the context of failing immunosurveillance, and stem cells occupy immune privileged microenvironments. Recent evidence demonstrates that CSCs borrow immune privilege from their normal counterparts. However, low doses of doxorubicin can target CSCs by restoring anticancer immunity.
Autocommentary
Current anticancer therapies typically use broadly toxic drugs. Combination chemotherapy is often effective at initially eliminating the majority of tumor cells; however, rare tumor-initiating cancer stem cells (CSCs) resist these therapies. CSCs retain or hijack normal stem cell properties. Stemness combines the ability to perpetuate a cell lineage by producing a large mass of more differentiated cells while maintaining a rare, self-renewing population protected from injuries that kill other cells.
An underappreciated stem cell protective property is the occupation of immune privileged microenvironments. For example, mesenchymal stem cells evade the immune system to the point that even interspecies transplantation is possible.Citation1 While not this extensive in hematopoietic stem cells (HSCs), evidence indicates HSCs evade the immune system and occupy immune privileged sites. The immune checkpoint Pd-l1 (Cd274), which is frequently associated with immune escape in cancer, is expressed on HSCs and improves allogeneic transplant capability.Citation2 Similarly, Cd47 is expressed on HSCs and certain cancer cells, which enables evasion of phagocytosis by macrophages.Citation3 Specialized T regulator cells have been shown to provide HSCs with immune privilege.Citation4 It has been hypothesized that HSCs generally express low levels of surface immune suppressors, which could be increased during times of stress or expansion to reinforce immune privilege and protect these rare but essential cells.Citation2
Unfortunately, CSCs can also borrow immune privilege from their normal stem cell counterparts. Immune resistance and escape arise from squamous cell carcinoma stem cells.Citation5 These tumor-initiating cells are resistant to T cell immunotherapy and subsequently form the root of relapse. Similarly, leukemia stem cells (LSCs) in acute myeloid leukemia (AML) have been functionally defined by their capacity for immune evasion irrespective of traditionally defined LSC-specific cell surface marker expression.Citation6 Specifically, AML cells lacking expression of a critical mediator of anti-cancer immunity, KLRK1 (best known as NKG2D), had the stemness properties of LSCs whereas those expressing NKG2D were recognized and killed by natural killer cells. Overall, these studies demonstrate a strong link between CSCs and immune escape.
Our recent work also highlights the powerful role CSCs can play in immune escape. The Wnt and PI3 K (phosphatidylinositol 3-kinase)/Akt pathways cooperatively interact to drive stem cell self-renewal and, when constitutively overactivated, the transformation of HSCs into LSCs.Citation7,Citation8 Both pathways also play a critical role in resistance to immunotherapy.Citation9 Given these roles, we sought to specifically target the interaction between them, specifically the activation of Ctnnb1 (best known as β-catenin) by Akt. High-throughput screening surprisingly identified doxorubicin (DXR), a highly toxic chemotherapy when used at typical clinical doses, as the top screen ‘hit’. Importantly, DXR’s on-target effect occurred well below the generally toxic dose. Consequently, we repurposed DXR as a targeted therapy to inhibit Akt-activated β-catenin.
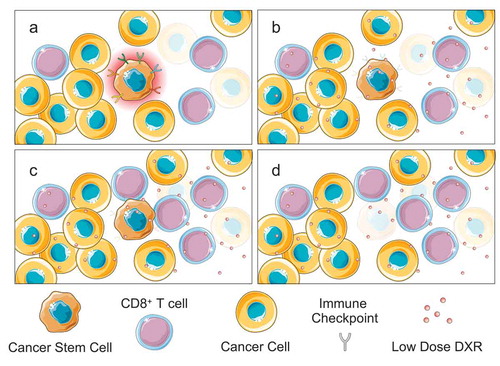
Transplantation experiments into immunocompromised mice found that low dose DXR could inhibit the establishment of leukemia from patient samples that had chemoresistant LSCs but had no effect on those lacking these cells. These and other experiments demonstrated that low dose DXR has an LSC autonomous targeting effect. However, investigating the broader mechanism in the full context of leukemia development and progression in immunocompetent mice revealed a striking role for the immune system in mediating low dose DXR’s LSC targeting ability (). Gene expression analysis revealed that stem cell/self-renewal related pathways were not the only or even predominant pathways altered by low dose DXR treatment. Instead, pathways relating to the immune system, particularly T cell pathways responsible for anticancer activity, were induced specifically with low but not high dose DXR treatment. Importantly, LSCs normally expressed multiple immune checkpoint genes, which were induced by Akt-activated β-catenin. By inhibiting Akt:β-catenin interaction in LSCs, low dose DXR treatment reduced expression of immune checkpoints. Furthermore, functional studies found that low dose DXR’s targeting of LSCs was largely abolished in the absence of CD8+ T cells. Overall, low dose DXR’s ability to target LSCs was found to be largely due to its LSC-intrinsic effect on the expression of multiple IC genes combined with an extrinsic effect of preserving and even stimulating T cell responses against LSCs.
Figure 1. Low-dose doxorubicin (DXR) overcomes immunoevasion by cancer stem cells (CSCs). A) CSCs occupy immune privileged sites and express diverse immune checkpoints, which protects them from elimination by T cells. B) Low dose DXR inhibits immune checkpoint expression driven by Akt-activated Ctnnb1 (β-catenin). C) Now lacking their immunosuppressive shield, CSCs are exposed to anticancer T cell activity and selectively eliminated (d).
Conventional chemotherapy and targeted anticancer agents can have largely unappreciated but positive immunological side effects.Citation10 For instance, they can increase the immunogenicity of cancer cells and inhibit immunosuppressive tumor microenvironments. In laboratory experiments, several chemotherapy drugs, particularly anthracyclines such as DXR, are known to induce immunogenic cell death (ICD). Unlike most forms of regulated cell death, ICD stimulates an immune response to dying cells – clearly an attractive prospect if the dying cell is malignant. Theoretically, one could induce cell death in a subset of cancer cells and exploit the ICD response to systemically eliminate cancer. However, until recently, anticancer drug development ignored any potential role for the immune system, and testing drugs in immunocompromised mouse models (a standard step in the process) fails to identify candidates that might function by stimulating the immune system. Any positive immunological side effects of typical chemotherapy and targeted drugs were merely fortuitous, and these drugs have not been optimized to account for such theoretical effects. Conversely, cancer is increasing recognized not simply as a cell-autonomous disease but as a failure of anti-cancer immunosurveillance. That CSCs, already known for their chemoresistance, can also be immunotherapy resistant complicates an already challenging situation. The need to better understand existing drugs and, more importantly, to develop new therapies accounting for the critical role of anticancer immunity particularly as it relates to CSCs will be essential to preventing treatment failure and relapse.
Acknowledgments
This research was supported by Stowers Institute for Medical Research (SIMR), Children’s Mercy Kansas City, Braden’s Hope for Childhood Cancer, the Leukemia & Lymphoma Society (LLS), Kansas Bioscience Authority, Hall Family Foundation, and University of Kansas Cancer Center (KUCC) National Cancer Institute Cancer Center Support Grant (NCI-CCSG) P30 CA168524 and used the KUCC Lead Development and Optimization Shared Resource.
References
- Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:1–3. doi:10.1002/stem.269.
- Zheng J, Umikawa M, Zhang S, Huynh HD, Silvany R, Chen BPC, Chen L, Zhang CC. Ex vivo expanded hematopoietic stem cells overcome the MHC barrier in allogeneic transplantation. Cell Stem Cell. 2011;9:119–130. doi:10.1016/j.stem.2011.06.003.
- Jaiswal S, Jamieson CHM, Pang WW, Park CY, Chao MP, Majeti R, Traver D, Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi:10.1016/j.cell.2009.05.046.
- Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, Gao W, Saito TI, Lo Celso C, Tsuyuzaki H, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–219. doi:10.1038/nature10160.
- Miao Y, Yang H, Levorse J, Yuan S, Polak L, Sribour M, Singh B, Rosenblum MD, Fuchs E. Adaptive immune resistance emerges from tumor-initiating stem cells. Cell. 2019;177:1172–1186 e1114. doi:10.1016/j.cell.2019.03.025.
- Paczulla AM, Rothfelder K, Raffel S, Konantz M, Steinbacher J, Wang H, Tandler C, Mbarga M, Schaefer T, Falcone M, et al. Absence of NKG2D ligands defines leukaemia stem cells and mediates their immune evasion. Nature. 2019;572:254–259. doi:10.1038/s41586-019-1410-1.
- Perry JM, He XC, Sugimura R, Grindley JC, Haug JS, Ding S, Li L. Cooperation between both Wnt/{beta}-catenin and PTEN/PI3K/Akt signaling promotes primitive hematopoietic stem cell self-renewal and expansion. Genes Dev. 2011;25:1928–1942. doi:10.1101/gad.17421911.
- Perry JM, Tao F, Roy A, Lin T, He XC, Chen S, Lu X, Nemechek J, Ruan L, Yu X, et al. Overcoming Wnt-beta-catenin dependent anticancer therapy resistance in leukaemia stem cells. Nat Cell Biol. 2020. doi:10.1038/s41556-020-0507-y.
- Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi:10.1016/j.cell.2017.01.017.
- Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714. doi:10.1016/j.ccell.2015.10.012.
