Abstract
Background: Parapneumonic effusions in children are usually associated with pneumococcal infections. In Greece, the 7-valent pneumococcal conjugate vaccine was replaced by higher-valent pneumococcal conjugate vaccines (PCVs); 10-valent was introduced in May 2009 and 13-valent (PCV13) in June 2010. Since July 2010, PCV13 has been the most commonly used PCV. In a study conducted at the University General Hospital of Larissa, Central Greece, from January 2012 to January 2016, 85.7% of children born after the implementation of PCV13 and aged 24–59 months had received the complete series (3 + 1 immunization schedule) of PCV13.
Methods: We studied all paediatric community-acquired pneumonia cases with empyema hospitalized at the University General Hospital of Larissa from January 2008 to January 2016.
Results: There were 30 cases of parapneumonic empyema. Among 27 empyema cases of known aetiology, 19 (70.4%) were due to Streptococcus pneumoniae (identifiable serotypes 3, 19A, 7F, and 9N/L). After September 2011, no more cases caused by serotypes 7F and 19A were observed, whereas serotype 3 emerged as the predominant pathogen of pneumococcal empyema (9 of 11 cases). Serotype 3 continued to cause empyema despite vaccination with PCV13 either fully with a 3 + 1 schedule (n = 3) or with one booster dose at the age of 21 months (n = 1).
Conclusion: In Central Greece during the first five years of high coverage with PCV13, serotype 3 was the only PCV13 serotype that clearly persisted in children with empyema.
Introduction
Community-acquired pneumonia (CAP) commonly occurs in children. Parapneumonic effusion is one of the complications of paediatric CAP.[Citation1] Parapneumonic effusions in children are usually associated with Streptococcus pneumoniae infections and they may progress to empyema.[Citation2,Citation3] Most of the cases of pneumococcal CAP with pleural effusion occur at ages 2–5 years.[Citation4–8]
The introduction of 7-valent pneumococcal conjugate vaccine (PCV7; capsular antigens of S. pneumoniae serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) led to a substantial decrease in pneumonia incidence.[Citation9,Citation10] However, PCV7 was unable to restrain the incidence of CAP complicated with pleural effusion.[Citation5,Citation6,Citation8] Recently, there has been increased interest towards the introduction of higher-valent PCVs, 10-valent (PCV10; serotypes 1, 5, 7F in addition to the serotypes included in PCV7) and 13-valent (PCV13; serotypes 1, 3, 5, 6A, 7F, and 19A in addition to the serotypes included in PCV7). PCV10 and PCV13 include serotypes recently known to be responsible for an increasing number of CAP cases, including those complicated with pleural effusion and empyema.[Citation11] The potential impact of PCV13 compared to PCV10 should be higher, as PCV13 contains additional serotypes, including serotype 3 and 19A, which are known to be frequently responsible for a number of CAP cases. So far, there is limited information on the impact of the higher-valent PCVs on the incidence of CAP complicated with pleural effusion, especially as these cases are more frequent in older toddlers.[Citation6,Citation7] Published early data from Spain and France with detailed information on pneumococcal serotypes cover the 1st and/or the 2nd year after the implementation of PCV13.[Citation12,Citation13]
In Greece, PCV7 became available in October 2004 and was incorporated into the national immunization program (NIP) in January 2006. Later, PCV10 was introduced to our country in May 2009 and PCV13 in June 2010. Both were included in the NIP in a 3 + 1 schedule and reimbursed by 100%. Since July 2010, PCV13 has been the most commonly used PCV.[Citation14]
In January 2011, extra doses of PCV13 were recommended by the NIP for those who were aged less than 60 months and had been previously vaccinated with PCV7. Specifically, two doses of PCV13 were recommended for ages 12–23 months, whereas one dose for ages 24–59 months.
The University General Hospital of Larissa (UGHL) serves as the academic, tertiary care referral centre for the broader area of Central Greece, which has a population of approximately 1,000,000 inhabitants. In a case-control study conducted in our Department encompassing the period January 2012–January 2016, a control group of 42 children born after the implementation of PCV13 and aged 24–59 months was investigated. It was found that 36 (85.7%) of the 42 children had received the complete series (3 + 1 immunization schedule) of PCV13 [authors’ unpublished data]. The aim of the present prospective observational study was to evaluate the etiologic agents of CAP cases with empyema in hospitalized children at the UGHL from January 2008 to January 2016.
Methods
From 1 January 2008 to 31 January 2016, we studied consecutive children hospitalized at the UGHL with the diagnosis of parapneumonic empyema, which was based on the complete pleural fluid evaluation. Pleural fluid specimen was obtained from children with suspected bacterial pneumonia by thoracentesis with or without chest tube placement. Thoracentesis and drainage of the parapneumonic effusion were considered in moderate to large in size effusions, taking also into consideration the child’s degree of respiratory compromise. In the present study, the research conformed to the Helsinki Declaration and to local legislation. The research protocol was approved by the Ethics Committee of the UGHL. The data were analysed anonymously.
Empyema definition
Empyema was considered to be present if there was consolidation and a pleural effusion on the chest radiograph combined with 1 or more of the following pleural fluid findings: cloudy or purulent appearance; white blood cell count >50,000 × 109 cells/L; pH <7.1; lactate dehydrogenase level >1000 IU/L; glucose level <40 mg/dL; positive Gram stain and/or culture and/or polymerase chain reaction (PCR) results.
Microbiologic investigations
Blood and pleural fluid specimens were cultured at the Laboratory of Microbiology of UGHL by standard culture method. A separate aliquot of pleural fluid was stored at −20 °C and was transported to the National Meningitis Reference Laboratory (NMRL), Athens, Greece (2008–2012) and/or the Immunology Laboratory, Anna Meyer Children’s Hospital (AMCH), Florence, Italy (2011–January 2016) for specific molecular studies, including S. pneumoniae and pneumococcal serotype identification by PCR as described previously.[Citation15,Citation16]
Capsule serotyping of S. pneumoniae isolates
Serotype determination of pneumococcal isolates was performed at the Laboratory of the Division of Paediatric Infectious Disease in Larissa by using Pneumotest-Latex and by the capsular swelling method using pneumococcal type/group and/or factor antisera from Statens Serum Institut (SSI, Copenhagen, Denmark).
Detection of Staphylococcus aureus leukocidin production
The S. aureus isolates were tested by PCR to detect the genes encoding the Panton–Valentine leukocidin production at the Department of Microbiology of UGHL as described previously.[Citation17]
PCR for other pathogens
All pleural fluid specimens that were found to be negative for S. pneumoniae were further tested by PCR using primers/probes for the presence of Haemophilus influenzae (type b and non typeable), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus spp., S. aureus, Neisseria meningitidis, Listeria monocytogenes, and Pseudomonas aeruginosa. In addition, at AMCH, specimens were tested for Escherichia coli, Klebsiella pneumoniae, and adenovirus DNA. In selected cases in which the pleural fluid specimen was 16S rRNA positive, but negative with all the primers tested, identification of the pathogen was obtained by performing 16S rRNA gene amplification and sequencing.
Statistical analysis
The prevalence of the pathogens during the study period was assessed using the χ2 for trend. p < 0.05 was considered statistically significant. The statistical analysis was carried out with the software product SPSS version 13.0.
Results
There were 30 CAP cases with empyema, which occurred in 2008–2009 (n = 10), 2010–2011 (n = 4), 2012–2013 (n = 7), and 2014–January 2016 (n = 9). The 30 cases had thoracentesis with (n = 29) or without (n = 1) chest tube placement. In 27 empyemas, the pathogen was identified (), whereas in three the aetiology remained unknown (2008–2009: n = 2 and 2012–2013: n = 1). The median age of the patients was 3.6 years (range 21 days to 8.2 years).
Table 1. Pathogens identified in 27 cases of parapneumonic empyema.
Pneumococcal vaccination
Between 2008 and 2011, among the 13 children who were aged 2 months or older, nine (69.2%) were fully vaccinated with PCV7 and two (15.4%) partially. None of these 11 children had received any dose of PCV10 or PCV13. Between January 2012 and January 2016, 13 (81.2%) of the 16 children with empyema were fully vaccinated with a PCV. Depending on the date of admission and the patients’ age at that time, eight children had received PCV7, three PCV13, one child PCV10 and one child PCV7 plus one dose of PCV13 as a booster. Overall, children born after March 2009 that were vaccinated with a PCV had received either a booster dose of PCV13 or the complete series of a higher-valent PCV.
Pneumococcal serotypes
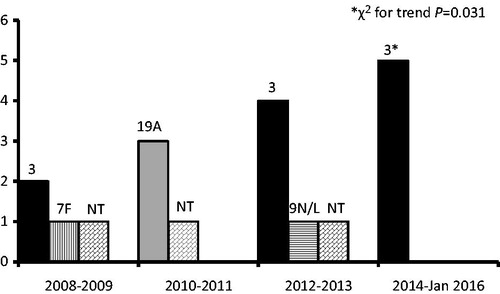
The S. pneumoniae serotypes were 3 (n = 11), 19A (n = 3), 7F (n = 1), and 9N/L (n = 1), whereas three specimens were nontypeable by the applied PCR assay. Serotype 3 cases were identified by PCR. Serotypes 3, 19A, and 7F accounted for the 93.8% of the specimens, in which S. pneumoniae was typeable by the applied techniques.
A significant increase in the cases of empyema due to S. pneumoniae serotype 3 was observed from January 2008 to January 2016 (χ2 for trend, p = 0.031) (). The median age of the children with serotype 3 empyema was 4.5 years (range 2.3–7.4 years). After September 2011, all but one of the pneumococcal cases with a PCR typeable serotype were due to serotype 3.
Figure 1. Pneumococcal serotypes causing parapneumonic empyema from January 2008 to January 2016. The 10-valent pneumococcal conjugate vaccine was introduced in May 2009 and the 13-valent in June 2010. NT, nontypeable by the applied polymerase chain reaction assay.
Nine (81.8%) of the 11 serotype 3 empyemas occurred after the implementation of PCV13 and more specifically in 2012 and onwards, when the uptake of PCV13 was high. During this period, four (44.4%) of the nine parapneumonic empyemas due to serotype 3 were observed in children who had been vaccinated with PCV13 either fully (3 children) or with one booster dose at the age of 21 months (1 child) (). They did not have any predisposing underlying condition and their medical history was free.
Table 2. Serotype 3 pneumococcal parapneumonic empyema in children vaccinated with 13-valent pneumococcal conjugate vaccine.
Discussion
The present study covered a relatively long time period and was carried out in a single academic, tertiary care referral centre by the same group of paediatricians and thoracic surgeons applying a consistent practice for the management of moderate to large parapneumonic effusions.
We were not able to identify any pneumococcal isolate belonging to serotype 14 or others included in PCV7, as the study took place at a time with high PCV7 coverage and consequent herd immunity.[Citation14]
Pneumococcal parapneumonic empyema cases caused by serotype 19A were observed during 2010 and 2011, when increased circulation of S. pneumoniae isolates belonging to serotype 19A was noted in Greece.[Citation14] In the following years, no further cases due to 19A were identified. In addition, after 2009 no case due to serotype 7F was observed. The vaccination with higher-valent PCVs, especially PCV13, appears to have contributed to this change.
In Central Greece during January 2012–January 2016 the absence of cases due to pneumococcal serotypes 7F and 19A coincided with the significant emergence of serotype 3 as the predominant etiologic agent of parapneumonic empyemas. Circulation of serotype 3 appears to remain relatively high.
We describe four cases of PCV13 failure against serotype 3, which suggests that the immune response for serotype 3 differs from that of the other PCV13 serotypes. Perhaps serotype 3 pneumococci are prone to causing parapneumonic effusions because they can produce high protective concentrations of capsular polysaccharide, overcome host immunity, and proliferate more efficiently than other pneumococcal serotypes.[Citation11] In addition, given the immunogenicity studies of PCV13 the immune responses for serotype 3 differ from that for the other serotypes: lower antibody titers and no or little booster effect (but no hyporesponsiveness).[Citation18–22] Nevertheless, the response for serotype 3 on opsonophagocytic activity assay is high when the infant series and toddler dose of PCV13 is used.[Citation19,Citation20]
There is a recent report on a three-year-old girl who developed a parapneumonic empyema caused by serotype 3, while she was fully vaccinated with PCV13, but in a 2 + 1 schedule.[Citation23] PCV13 failure to offer protection from parapneumonic effusion due to serotype 3 was also noted in a catch-up program with one dose of PCV13 administered after the 2nd year of life, whereas one child had received two doses, the first at the age of 21 months.[Citation24]
The failure of PCV13 in cases of parapneumonic empyema due to serotype 3 does not predict the inability of the vaccine to reduce the incidence of CAP cases due to serotype 3, as parapneumonic effusions represent only a subset of complicated pneumonias with an underlying mechanism which still remains unclear.
We did not find any case of parapneumonic empyema caused by serotype 1, especially before the use of PCV10 and PCV13. It is worth noting that we have applied molecular techniques that have high sensitivity in the identification of serotype 1 directly on pleural fluid specimen. A low prevalence of serotype 1 has been also observed in children in Utah with pneumococcal parapneumonic empyema during 2009 (4%) [Citation5] and in Ontario, Canada during 2009–2011 (2.9%).[Citation25]
The potential of the emerging non-PCV13 colonizing serotypes [Citation14,Citation26] to cause CAP with empyema remains to be seen in the future.
A limitation of the present study is the relatively small number of S. pneumoniae-PCR positive samples that were available for PCR serotyping. However, the identified serotypes 3, 19A, and 7F, are in line with most recent reports,[Citation5,Citation6,Citation11,Citation25] with the exception of serotype 1, which was absent in our study. The strength of the study is the clear illustration of a significant increase of serotype 3 cases and the failure of PCV13 to protect from the development of empyema due to serotype 3.
In conclusion, during the first five years of high coverage with PCV13, no more cases of parapneumonic empyemas caused by pneumococcal serotypes 7F and 19A were observed. However, during the same period, serotype 3 emerged as the predominant pathogen in empyemas. Serotype 3 was the only PCV13 serotype that clearly persisted and caused pneumococcal parapneumonic empyema in children. Failure of PCV13 was noted among children that had received either the complete series (3 + 1 immunization schedule) or one toddler dose. Continued surveillance will clarify further the PCV13 impact on CAP with pleural effusion, as well as the emergence of any replacement non-PCV13 serotypes.
Acknowledgements
We thank Drs. Chiara Azzari and Francesco Nieddu of the Immunology Laboratory, Anna Meyer Children’s Hospital, Florence, Italy and Georgina Tzanakaki of the National Meningitis Reference Laboratory, National School of Public Health, Athens, Greece for performing the molecular studies of the pleural fluid specimens.
Disclosure statement
The authors declare that they have not any commercial relationship or potential conflict of interest related to the submission. No funding was received for the study.
References
- Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25–e76.
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc. 2006;3:75–80.
- Sahn SA. Diagnosis and management of parapneumonic effusions and empyema. Clin Infect Dis. 2007;45:1480–1486.
- Resti M, Moriondo M, Cortimiglia M, et al. Community‐acquired bacteremic pneumococcal pneumonia in children: diagnosis and serotyping by real‐time polymerase chain reaction using blood samples. Clin Infect Dis. 2010;51:1042–1049.
- Blaschke AJ, Heyrend C, Byington CL, et al. Molecular analysis improves pathogen identification and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J. 2011;30:289–294.
- Strachan RE, Cornelius A, Gilbert GL, et al. Bacterial causes of empyema in children, Australia, 2007–2009. Emerging Infect Dis. 2011;17:1839–1845.
- Obando I, Camacho-Lovillo MS, Porras A, et al. Sustained high prevalence of pneumococcal serotype 1 in paediatric parapneumonic empyema in southern Spain from 2005 to 2009. Clin Microbiol Infect. 2012;18:763–768.
- Lin TY, Hwang KP, Liu CC, et al. Etiology of empyema thoracis and parapneumonic pleural effusion in Taiwanese children and adolescents younger than 18 years of age. Pediatr Infect Dis J. 2013;32:419–421.
- Jardine A, Menzies RI, McIntyre PB. Reduction in hospitalizations for pneumonia associated with the introduction of a pneumococcal conjugate vaccination schedule without a booster dose in Australia. Pediatr Infect Dis J. 2010;29:607–612.
- Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155–163.
- Yu J, Salamon D, Marcon M, et al. Pneumococcal serotypes causing pneumonia with pleural effusion in pediatric patients. J Clin Microbiol. 2011;49:534–538.
- Picazo J, Ruiz-Contreras J, Casado-Flores J, et al. Expansion of serotype coverage in the universal pediatric vaccination calendar: short-term effects on age- and serotype-dependent incidence of invasive pneumococcal clinical presentations in Madrid, Spain. Clin Vaccine Immunol. 2013;20:1524–1530.
- Angoulvant F, Levy C, Grimprel E, et al. Early impact of 13-valent pneumococcal conjugate vaccine on community-acquired pneumonia in children. Clin Infect Dis. 2014;58:918–924.
- Grivea IN, Priftis KN, Giotas A, et al. Dynamics of pneumococcal carriage among day-care center attendees during the transition from the 7-valent to the higher-valent pneumococcal conjugate vaccines in Greece. Vaccine. 2014;32:6513–6520.
- Xirogianni A, Tsolia M, Voyiatzi A, et al. Diagnosis of upper and lower respiratory tract bacterial infections with the use of multiplex PCR assays. Diagnostics. 2013;3:222–231.
- Azzari C, Moriondo M, Indolfi G, et al. Realtime PCR is more sensitive than multiplex PCR for diagnosis and serotyping in children with culture negative pneumococcal invasive disease. PLoS One. 2010;19:e9282.
- Chini V, Petinaki E, Foka A, et al. Spread of Staphylococcus aureus clinical isolates carrying Panton–Valentine leukocidin genes during a 3-year period in Greece. Clin Microbiol Infect. 2006;12:29–34.
- Yeh SH, Gurtman A, Hurley DC, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in infants and toddlers. Pediatrics. 2010;126:e493–e505.
- Snape MD, Klinger CL, Daniels ED, et al. Immunogenicity and reactogenicity of a 13-valent-pneumococcal conjugate vaccine administered at 2, 4, and 12 months of age: a double-blind randomized active-controlled trial. Pediatr Infect Dis J. 2010;29:e80–e90.
- Kieninger DM, Kueper K, Steul K, et al. Safety, tolerability, and immunologic noninferiority of a 13-valent pneumococcal conjugate vaccine compared to a 7-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in Germany. Vaccine. 2010;28:4192–4203.
- Esposito S, Tansey S, Thompson A, et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine compared to those of a 7-valent pneumococcal conjugate vaccine given as a three-dose series with routine vaccines in healthy infants and toddlers. Clin Vaccine Immunol. 2010;17:1017–1026.
- Vanderkooi OG, Scheifele DW, Girgenti D, et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine in healthy infants and toddlers given with routine pediatric vaccinations in Canada. Pediatr Infect Dis J. 2012;31:72–77.
- Madhi F, Godot C, Bidet P, et al. Serotype 3 pneumococcal pleural empyema in an immunocompetent child after 13-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2014;33:545–546.
- Antachopoulos C, Tsolia MN, Tzanakaki G, et al. Parapneumonic pleural effusions caused by Streptococcus pneumoniae serotype 3 in children immunized with 13-valent conjugated pneumococcal vaccine. Pediatr Infect Dis J. 2014;33:81–83.
- Slinger R, Hyde L, Moldovan I, et al. Direct Streptococcus pneumoniae real-time PCR serotyping from pediatric parapneumonic effusions. BMC Pediatr. 2014;14:189.
- Ahn JG, Choi SY, Kim DS, et al. Changes in pneumococcal nasopharyngeal colonization among children with respiratory tract infections before and after use of the two new extended-valency pneumococcal conjugated vaccines. Infect Dis. 2015;47:385–392.
