?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background: We aimed to investigate whether very low-level HIV-1 viraemia (VLLV) <20 copies/mL in HIV-1-infected patients on antiretroviral therapy (ART) whose VL was <20 copies/mL, was associated with a subsequent VL > 20 copies/mL.
Methods: VLLV was defined as VL <20 copies/mL and positive HIV-1-PCR. We compared patients with positive and negative HIV-1-PCRs <20 copies/mL at two time points, T0 and T1, after 21st of January 2016. Factors associated with a VLLV and subsequent VL >20 copies/mL were identified by logistic regression models.
Results: Of 1341 participants at T0, 958 (71.4%) had a negative and 383 patients (28.6%) had positive HIV-RNA PCR signal during VL < 20 copies/mL. The negative relative to the positive signal at T0 was independently associated with dolutegravir (DTG) mono and/or DTG-lamivudine dual therapy (compared to nevirapine), a pre-ART-VL of 1000–9999 copies/mL (compared to ≥100,000 copies/mL), and each additional year of virologic suppression. Having a virolologic outcome at T1 of 20 copies/mL was independently associated with prior positive signal at T0. (OR = 2.291, 95% CI = 1.457–3.601, p value < .001), a past ART interruption, and a change in ART regimen during follow-up. Each additional year of virologic suppression was independently associated with a lower risk for a subsequent VL ≥ 20 copies/mL.
Conclusions: A positive HIV-1 RNA PCR <20 copies/mL at T0, was associated with a subsequent VL ≥ 20 copies/mL at T1. This was not a rare phenomenon among patients with VL <20 copies/mL. In most patients with a positive PCR signal, this was not followed by a clinically relevant HIV-1 viraemia, defined as ≥ 200 copies/mL.
Introduction
Current guidelines state that with antiretroviral therapy (ART) optimal virologic control is achieved if viral load (VL) is below the lower limit of quantification (LLoQ) of the assay used (generally VL <50 copies/mL) [Citation1]. Observational studies showed that maintaining VL < 50 copies/mL predicts virologic, immunologic and clinical benefits [Citation2]. In recent years, ultrasensitive VL assays made it possible to detect HIV-1 viraemia at very low levels. Previous research produced conflicting results on the consequences of very low-level HIV-1 viraemia (VLLV) of <50 copies/mL. Some studies found VLLV to be significantly associated with a subsequent virologic rebound of >50 copies/mL [Citation3–8], >200 copies/mL [Citation6,Citation9], and >400 copies/mL [Citation4,Citation6], whereas others failed to acknowledge an association between VLLV and subsequent virologic failure [Citation10–12]. The development of sensitive VL assays has made physicians aware of the relatively high rate of ongoing VLLV in ART-treated patients without specific knowledge of its clinical significance.
The Erasmus Medical Center uses The Cobas AmpliPrep/Cobas Taqman HIV-1 test v2.0 (Roche Diagnostics) with a lower limit of quantification (LLoQ) of 20 copies/mL. Since January 2016 an addition to the test result is provided whether a HIV-RNA PCR signal is still detectable below <20 copies/mL. A positive signal implicates the presence of 10–20 copies/mL with a detection rate of 95%, (95% confidence interval (CI)) [Citation13].
The aim of this study was to investigate the association between a positive HIV-1 RNA PCR signal of 10–20 copies/mL relative to a negative HIV-1 RNA PCR signal in HIV-positive patients on antiretroviral therapy (ART) with a VL <20 copies/mL, at an arbitrarily selected time point during ART (T0), and the development of a subsequent VL ≥20 copies/mL.
Methods
Study design: This was a single-centre, retrospective study among 2056 HIV-1-infected patients in care at the Erasmus Medical Center (MC) with available VL data from the 21st of January 2016 up to the 26th of April 2017.
Inclusion criteria: HIV-1-infected patients, aged ≥18 years and receiving ART, initiated before the study period, were included in the analysis if their first plasma VL measurement in the period from the 21st of January 2016 up to the 26th of March 2017 was below the LLoQ (i.e. VL <20 copies/mL). The ErasmusMC HIV cohort is part of the HIV Monitoring Foundation ATHENA cohort database, which contains records from 1997 onwards, from all HIV-infected patients receiving care at any of the 26 HIV treatment centres in the Netherlands [Citation14]. The ATHENA cohort database was approved by an institutional ethics review board, and from all patients, informed consent was obtained.
Data collection: Data were obtained during routine follow-up visits at the outpatient clinic with 6 months intervals. We collected two consecutive VL measurements (T0 and T1). T0 was defined as the moment of the first plasma VL measurement in the period from the 21st of January 2016 up to the 26th of March 2017. VL at T0 could be either be negative (i.e. HIV-1 RNA below LLoQ of 20 copies/mL and HIV-1 RNA not detected by PCR) or positive (i.e. HIV-1 RNA below the LLoQ of20 copies/mL and HIV-1 RNA detected by PCR). T1 was the second consecutive VL measurement with THREE possible results: negative, positive, or ≥20 copies/mL (i.e. HIV-1 RNA above the LLoQ). Demographic and clinical data were obtained from medical records.
Statistical analysis: Differences in patient, laboratory, and therapy characteristics between groups (i.e. VL negative or positive at T0 and VL <20 copies/mL or ≥20 copies/mL at T1) were primarily analysed using either the Chi-squared test or the Fisher’s exact test for categorical data, depending on the percentage of cells with an expected count less than 5. Only if this percentage exceeded 20, the Fisher’s exact test was used. Continuous variables were primarily analysed using the independent sample t-test; the Mann–Whitney U-test was used for data not normally distributed. Univariate and multivariate binary logistic regression models were used to identify factors that were associated with VL at T0 (i.e. negative or positive) and factors that were associated with VL at T1 (i.e. VL <20 copies/mL or VL ≥20 copies/mL). Variables with a p value < .100 in the primary analysis were entered into the multivariate models. In all analyses, a p value <.050 was considered to be indicative of a statistically significant association. All analyses were performed using IBM SPSS Statistics 21.
Results
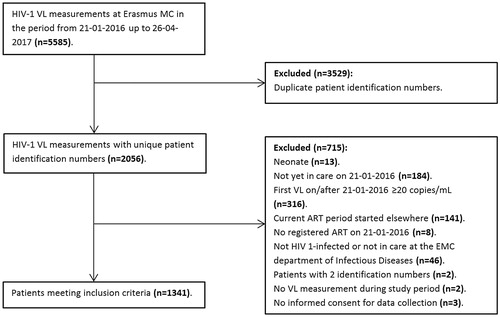
Study population During the period from the 21st of January 2016 up to the 26th of April 2017 5585 VL samples from 2056 patients were available. In this study, 715 patients did not fulfil the inclusion criteria for various reasons (), resulting in 1341 patients with a VL below the LLoQ that were eligible for analysis. An overview of baseline and follow-up characteristics of the 1341 patients included in the study is shown in . Most patients were male (74.6%), MSM (56.2%) and 44.7% were late presenters with a nadir CD4 cell count of <200/mm3. The majority of the patients (62.2%) were on a non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimen, 11.5% used a protease inhibitor (PI)-based regimen and 24.3% used a integrase inhibitor (INI) based regimen.
Table 1. Overview of baseline characteristics of patients included in the study.
The association between patient, laboratory, and therapy characteristics stratified by VL (i.e. negative or positive) at T0 for all patients (n = 1341) is shown in . At T0, 958 patients (71.4%) had an undetectable PCR signal (i.e. negative) and 383 patients (28.6%) had an detectable PCR <20 copies/mL and VL < 20 copies/mL (i.e. positive). VL with a negative HIV-1 PCR signal at T0 was independently associated with dolutegravit (DTG) monotherapy and DTG-lamivudine (3TC) dual therapy as ART regimen (compared to a nevirapine (NVP)-based regimen, p = .023), pre-ART-VL 1000–10,000 copies/mL (compared to VL ≥100,000 copies/mL, p value = .004), years of uninterrupted ART (p value < .001), and years of virologic suppression (p < .001). Gender, age, region of birth, risk group, years from HIV-1 diagnosis, nadir CD4+ cell count, T0 CD4+ cell count, virologic failure, and past ART interruption were not associated with VL at T0.
Table 2. Univariate and multivariate logistic regression results: patient, laboratory, and therapy characteristics, stratified by VL, that is, negative (n = 958) or positive (n = 383) at T0.
Narrowing our scope to patients with two available VL measurements, VL outcomes at T0 and T1 are shown in . From 358 patients with a positive HIV-1 PCR signal at T0, 49 (13.7%) developed a VL ≥20 copies/mL at T1. From those 49 patients, 5 (1.39%) reached levels of >200 copies/mL and 4 (1.12%) had >1000 copies/mL at T1, resulting in extra interventions. Whereas 47 (5.3%) patients out of a total of 891 patients with negative HIV-1 PCR signal at T0, had a VL ≥20 copies/mL at T1. Among these 47 patients, were 13 patients (1.45%) with VL >200 copies/mL at T1 and 10 (1.12%) with VL >1000 copies/mL.
Table 3. VL at T0 and T1 (all patients with 2 VL measurements).
In order to acknowledge the relative importance of the specification (i.e. negative or positive) of the previous unquantifiable VL measurement and to clarify which factors were independently associated with the development of a quantifiable VL (≥20 copies/mL) at T1 we performed a univariate and multivariate logistic regression analysis of patient, laboratory, and therapy characteristics stratified by VL at T1 (i.e. <20 or ≥20 copies/mL) for all patients with 2 available VL measurements (n = 1249) (). In univariate analysis, patients with an unquantifiable VL specified as positive (10–20 copies/mL) at T0 had a significantly higher risk for VL ≥20 copies/mL at T1 than patients with a negative HIV-1 RNA signal at T0 (OR = 2.848, 95% CI = 1.896–4.338, p value < .001).
Table 4. Univariate and multivariate logistic regression results: patient, laboratory, and therapy characteristics stratified by VL (i.e. <20 copies/mL or ≥20 copies/mL) at T1 (all patients).
Factors independently associated with VL ≥20 copies/mL at T1 according to the multivariate model, were a positive HIV-1 RNA PCR signal (10–20 copies/mL) at T0 (OR = 2.791 95% CI = 1.457–3.601, p value < .001), years of virologic suppression (OR = 0.909, 95% CI = 0.846–0.976, p value = .009), and a past ART interruption started on the patient’s initiative or imposed by the physician based on suspected lack of compliance (OR = 2.315, 95% CI = 1.293–4.146, p value = .005). A modification of ART regimen between T0 and T1 during follow-up was also significantly associated with VL ≥20 copies/mL at T1 (OR = 2.657, 95% CI = 1.671–4.226, p value < .001).
Discussion
This study confirmed that a positive HIV-1 RNA PCR signal and a VL below the LLoQ in HIV-1-infected patients treated with ART, is not a rare phenomenon. Out of all patients with 2 VL measurements available, 42.9% of patients had at least 1 VL <20 copies/mL specified as positive. DTG-mono ± lamivudine as ART regimen (relative to a NVP-based regimen), a pre-ART VL of <10,000 copies/mL (relative to a pre-ART VL ≥100,000 copies/mL), and each additional year of virologic suppression were independently associated with a significantly lower risk for an unquantifiable positive VL. Furthermore, our study demonstrated that a positive PCR signal at T0 was independently associated with a subsequent VL ≥20 copies/mL at T1 (OR = 2.291, 95% CI = 1.457–3.601, p value < .001).
In our HIV-1-infected population, 583 out of 1249 patients (46.7%), who initially all had a VL < 20 copies/mL at baseline, experienced HIV-1 viraemia to some extent (i.e. a VL 10–20 copies/mL specified as positive at T0 and/or T1, or a VL ≥20 copies/mL at T1) during follow-up. This is in accordance with other recent studies. The study by Henrich et al found in 33% of 778 single VL measurements detectable HIV-RNA that was below the LLoQ of 48 copies/mL [Citation6]. Maggiolo et al found in their cohort of 1214 patients during a mean follow-up of 378 days at each interval of 4 months approximately 70% of subjects with a VL <3 copies/mL at all sampling times. Their data suggested that the average steady-state VL is below 3 copies/mL; however, only 480 patients (40.6%) showed a steadily controlled viraemia < 3 copies/mL [Citation7]. In our population, 53.3% of patients had a steadily negative HIV-1 RNA PCR signal. The differences between the results of several studies could be explained by the frequency of monitoring VL during the follow-up and/or the LLoQ of the tests. The proportion of patients with VLLV below the LLoQ in our study is similar to the proportion found in other recent studies.
Research from most studies analysing low-level HIV-1 viraemia (LLV) in the range of 50 up to 1000 copies/mL [Citation9,Citation15–21], indicated that VLLV below the LLoQ with a detectable HIV-1-PCR is associated with subsequent virologic rebounds. Studies focusing on VLLV report conflicting results on the consequences of the presence HIV-1 viraemia <50 copies/mL [Citation3–8,Citation10–12,Citation18,Citation22]. The strength of the association between VLLV and subsequent viral rebound depends on the definitions of VLLV and virological rebound. Many studies observed a statistically significant association between a quantifiable VL <50 copies/mL and a subsequent virologic rebound of ≥50 copies/mL, whereas only few studies found a statistically significant result for higher levels of virologic rebound. We confirmed the association between a positive HIV-1 PCR signal in patients with a VL < LLoQ and a subsequent virological rebound of ≥20 copies/mL, adding to previous research that even HIV-1 viraemia at levels as low as <20 copies/mL was not a random phenomenon, and significantly associated with a subsequent virologic rebound.
Factors associated with a positive PCR signal when the VL is < LLoQ identified in previous studies, were duration of virologic suppression [Citation3,Citation4,Citation7,Citation10,Citation23], pre-ART-VL [Citation4,Citation7], and duration of ART usage [Citation3,Citation4]. The main difference between previous research in general and our study, was the more strict definition of a detectable VL (i.e. VL <20/copies/mL instead of <50 copies/mL). In previous studies, PI-based ART regimens were reported to be significantly associated with developing a quantifiable VL, possibly due to the result of limited potency of PI-based ART regimens and/or by a potential bias for prescribing PI-based regimens for patients who previously experienced virologic failure or adherence problems [Citation9,Citation20]. In our study, PI-based ART regimens were not an independent risk factor for VLLV (specified as positive), as all multivariate analyses showed no differences between PI-based ART regimens and the reference category of NNRTI + NRTIs. Perhaps this is a consequence of decreasing side effects and easier dosing regimens of modern PI regimens [Citation24].
Several studies observed an association between NNRTI-based ART regimens and the likelihood of achieving either an unquantifiable VL (specified as negative) or an unquantifiable VL in an assay having a LL0Q of 2.5–3 copies/mL [Citation7,Citation25,Citation26], depending on the VL assay used. Haïm-Boukobza et al., comparing 2 NNRTI-based ART regimens, found that NVP + NRTIs, relative to efavirenz (EFV)+NRTIs, was independently associated with a VL <1 copy/mL (OR = 2.85, 95% CI = 1.4–6.1, p value = .005) [Citation23]. In contrast, in our study, we did not observe a difference in potency to achieve a VL < 20 copies/mL and a negative HIV-1 RNA PCR for NVP + NRTIs and EFV + NRTIs, although the numbers of patients in our analysis were sufficient (397 patients on NVP + NRTIs and 188 patients on EFV + NRTIs). Haïm-Boukobza et al. used a different outcome measure by comparing patients with a VL <1 copy/mL to patients with a VL of 1–49 copies/mL, which could account for these contradictory results.
The category DTG-mono ± lamivudine was significantly associated with a negative HIV-1 RNA PCR at T0 in multivariate analysis of all patients. This was probably the result of inclusion of a number of (preselected) participants of the DTG-monotherapy maintenance study [Citation27], rather than the ART regimen itself.
In our study, pre-ART VL was significantly associated with the HIV-1 PCR signal at T0. Patients having a pre-ART VL of 1000–9999 copies/mL (compared to a pre-ART VL ≥100,000 copies/mL) were significantly less likely to have a positive HIV-1 RNA PCR signal. This is consistent with the results of Maldarelli et al., who concluded that pre-ART VL accounts for 17% of the variability in stable LLV in patients on ART [Citation28].
We also found a strong association between shorter duration of virologic suppression and having a positive HIV-1 RNA PCR signal at T0. This finding could be a result of ongoing viral replication due to (a combination of) several factors such as a large latent reservoir, a lack of potency of ART and/or suboptimal therapy adherence [Citation29]. This ongoing viral replication could result in a VL below the LLoQ (i.e. an unquantifiable VL with a positive HIV-1 PCR signal) or a VL just above the LLoQ (i.e. a viral blip) [Citation9,Citation20].
Strengths and limitations
The strength of our study was the relative large sample size, representative of a divers, “real-life” HIV-1-infected population. A wide variety of ART regimens, including modern regimens (e.g. DTG + NRTIs), were used as opposed to previous research that focused mainly on PI- or NNRTI-based regimens. All VL measurements were performed at the Erasmus MC using the Cobas AmpliPrep/Cobas Taqman HIV-1 test v2.0 assay.
Our study also had several limitations. The duration of baseline ART regimen prior to the 21st of January 2016 may have underestimated the duration of some antiretroviral drugs, because any change in the ART regimen was regarded as the start of a new regimen. Modernization of a twice-daily regimen to a once-daily regimen, for example by altering zidovudine combined with lamivudine (3TC) in tenofovir disoproxil fumarate combined with emtricitabine, was regarded as a new ART regimen even though the third antiretroviral drug was continued. Among the group of DTG-mono ± 3TC the highest percentage of patients changed to a different ART regimen (77 patients, 81.1%). Although a large number of patients experienced an ART change in this group, the actual moment of switch was rather late during the follow-up; the mean duration of DTG-monotherapy ±3TC as ART regimen during follow-up was 339 days, whereas the mean duration for all ART regimens was 380 days.
Our study did not provide data on therapy compliance.
Conclusion
VLLV was not a rare phenomenon among patients with a VL below the LLoQ. A positive HIV-1 RNA PCR signal in patients with a VL <20 below the LLoQ, was associated with a subsequent VL ≥20 copies/mL. This reflects suboptimal virologic control and could indicate virologic failure. Identifying these patients at an early stage could be an opportunity for a medical intervention to prevent virologic failure. However, most factors that were associated with VLLV, cannot be influenced by medical interventions.
While we must recognize the increased risk of developing a quantifiable VL in patients with VLLV, we must also conclude that performing additional medical interventions (e.g. more frequent VL measurements or changes in ART regimen) in this group of patients would lead to many unnecessary interventions, because the vast majority of patients presenting with VLLV do not develop a clinically significant HIV-1 viraemia. Extra interventions come at a cost both financially and in patient well-being. We therefore suggest that an VLLV should in itself not warrant additional medical interventions.
Acknowledgements
We gratefully acknowledge the contribution all physicians, nurses and patients.
Disclosure statement
None of the authors has any conflicts of interest to declare.
References
- Gunthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191–210.
- Mocroft A, Phillips AN, Gatell J, et al. Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet. 2007;370:407–413.
- Alvarez Estevez M, Chueca Porcuna N, Guillot Suay V, et al. Quantification of viral loads lower than 50 copies per milliliter by use of the Cobas AmpliPrep/Cobas TaqMan HIV-1 test, version 2.0, can predict the likelihood of subsequent virological rebound to >50 copies per milliliter. J Clin Microbiol. 2013;51:1555–1557.
- Doyle T, Smith C, Vitiello P, et al. Plasma HIV-1 RNA detection below 50 copies/mL and risk of virologic rebound in patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2012;54:724–732.
- Gianotti N, Galli L, Salpietro S, et al. Virological rebound in human immunodeficiency virus-infected patients with or without residual viraemia: results from an extended follow-up. Clin Microbiol Infect. 2013;19:E542–E544.
- Henrich TJ, Wood BR, Kuritzkes DR. Increased risk of virologic rebound in patients on antiviral therapy with a detectable HIV load <48 copies/mL. PLoS One. 2012;7:e50065.
- Maggiolo F, Callegaro A, Cologni G, et al. Ultrasensitive assessment of residual low-level HIV viremia in HAART-treated patients and risk of virological failure. J Acquir Immune Defic Syndr. 2012;60:473–482.
- Widdrington J, Payne B, Medhi M, et al. The significance of very low-level viraemia detected by sensitive viral load assays in HIV infected patients on HAART. J Infect. 2011;62:87–92.
- Leierer G, Grabmeier-Pfistershammer K, Steuer A, et al. A single quantifiable viral load is predictive of virological failure in human immunodeficiency virus (hiv)-infected patients on combination antiretroviral therapy: The Austrian HIV Cohort Study. Open Forum Infect Dis. 2016;3:ofw089.
- Charpentier C, Landman R, Laouenan C, et al. Persistent low-level HIV-1 RNA between 20 and 50 copies/mL in antiretroviral-treated patients: associated factors and virological outcome. J Antimicrob Chemother. 2012;67:2231–2235.
- Gianotti N, Galli L, Racca S, et al. Residual viraemia does not influence 1 year virological rebound in HIV-infected patients with HIV RNA persistently below 50 copies/mL. J Antimicrob Chemother. 2012;67:213–217.
- Teira R, Vidal F, Munoz-Sanchez P, et al. Very low level viraemia and risk of virological failure in treated HIV-1-infected patients. HIV Med. 2017;18:196–203.
- Cobb BR, Vaks JE, Do T, et al. Evolution in the sensitivity of quantitative HIV-1 viral load tests. J Clin Virol. 2011;52 Suppl 1:S77–S82.
- van Sighem AI, Boender TS, Wit FWNM, et al. HIV Monitoring report 2017. Human Immunodeficiency Virus (HIV) infection in The Netherlands. Amsterdam: Stichting HIV Monitoring; 2017.
- Boillat-Blanco N, Darling KE, Schoni-Affolter F, et al. Virological outcome and management of persistent low-level viraemia in HIV-1-infected patients: 11 years of the Swiss HIV Cohort Study. Antivir Ther. 2014;20:165–175.
- Elvstam O, Medstrand P, Yilmaz A, et al. Virological failure and all-cause mortality in HIV-positive adults with low-level viremia during antiretroviral treatment. PLoS One. 2017;12:e0180761.
- Geretti AM, Smith C, Haberl A, et al. Determinants of virological failure after successful viral load suppression in first-line highly active antiretroviral therapy. Antivir Ther. 2008;13:927–936.
- Hofstra LM, Mudrikova T, Stam AJ, et al. Residual viremia is preceding viral blips and persistent low-level viremia in treated HIV-1 patients. PLoS One. 2014;9:e110749.
- Laprise C, de Pokomandy A, Baril JG, et al. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis. 2013;57:1489–1496.
- Vancoillie L, Demecheleer E, Callens S, et al. Markers associated with persisting low-level viraemia under antiretroviral therapy in HIV-1 infection. J Antimicrob Chemother. 2014;69:1098–1103.
- Vandenhende MA, Perrier A, Bonnet F, et al. Risk of virological failure in HIV-1-infected patients experiencing low-level viraemia under active antiretroviral therapy (ANRS C03 cohort study). Antivir Ther. 2015;20:655–660.
- Pernas B, Grandal M, Pertega S, et al. Any impact of blips and low-level viraemia episodes among HIV-infected patients with sustained virological suppression on ART?. J Antimicrob Chemother. 2016;71:1051–1055.
- Haim-Boukobza S, Morand-Joubert L, Flandre P, et al. Higher efficacy of nevirapine than efavirenz to achieve HIV-1 plasma viral load below 1 copy/mL. AIDS. 2011;25:341–344.
- Sungkanuparph S, Overton ET, Seyfried W, et al. Intermittent episodes of detectable HIV viremia in patients receiving nonnucleoside reverse-transcriptase inhibitor-based or protease inhibitor-based highly active antiretroviral therapy regimens are equivalent in incidence and prognosis. Clin Infect Dis. 2005;41:1326–1332.
- Bonora S, Nicastri E, Calcagno A, et al. Ultrasensitive assessment of residual HIV viraemia in HAART-treated patients with persistently undetectable plasma HIV-RNA: a cross-sectional evaluation. J Med Virol. 2009;81:400–405.
- Palmisano L, Giuliano M, Nicastri E, et al. Residual viraemia in subjects with chronic HIV infection and viral load < 50 copies/mL: the impact of highly active antiretroviral therapy. AIDS. 2005;19:1843–1847.
- Wijting I, Rokx C, Boucher C, et al. Dolutegravir as maintenance monotherapy for HIV (DOMONO): a phase 2, randomised non-inferiority trial. Lancet HIV. 2017;4:e547.
- Maldarelli F, Palmer S, King MS, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3:e46.
- Darcis G, Bouchat S, Kula A, et al. Reactivation capacity by latency-reversing agents ex vivo correlates with the size of the HIV-1 reservoir. AIDS. 2017;31:181–189.