Abstract
Background: Skin and soft tissue infections (SSTIs) are increasing. Frequent over- and under-treatment has been reported, including non-purulent SSTIs where cases demanding surgery or broad-spectrum therapy often are hard to identify. Our aim was to measure the predictive power of a modified severity score and use it to identify areas of improvement in antimicrobial therapy of non-purulent SSTIs.
Methods: We prospectively included adult patients admitted to hospital with non-purulent SSTIs. A modified Dundee score at admission was calculated retrospectively, and associations between severity and outcomes were analysed. We evaluated appropriateness of treatment in relation to severity scores, and assessed adverse effects of broad-spectrum therapy.
Results: We included 200 cases with cellulitis and 19 cases with necrotising soft tissue infections (NSTIs). Thirty-two per cent were categorised as severity class I, 15% as class II, 28% as class III and 25% as class IV (most severe). In class I, 66 out of 69 cases did not have a complicated course. All but one NSTI case were identified by the class IV criteria. Over-treatment was common and mostly seen in class I. Broad-spectrum antibiotics or clindamycin use was associated with an increased risk of diarrhoea. Prolonged treatment (>14 days) was associated with age, severity and surgery.
Conclusions: The modified Dundee score proved valuable in identifying those with the lowest risk of complication and the most severe infections, and could serve as a useful clinical tool in the emergency department. Frequent over-treatment and associated adverse effects were confirmed, underscoring the need for improved risk assessment.
Introduction
Skin and soft tissue infections (SSTIs) are heterogeneous and among the most common causes of hospitalisation for antibiotic treatment [Citation1,Citation2]. The global burden of SSTIs is increasing, including non-purulent SSTIs such as cellulitis [Citation3–5]. A major problem in the management of non-purulent SSTIs is frequent over-treatment with use of broad-spectrum antibiotics, even in mild cases, partly reflecting difficulties in establishing the microbial aetiology [Citation5]. Cases of moderate severity are also prone to over-treatment, as they may be hard to discern from early phases of necrotising soft tissue infections (NSTIs) [Citation6]. Concerningly, at the severe end of the disease spectrum under-treatment is not uncommon [Citation7,Citation8]. Both over- and under-treatment often represent inadequate adherence to published guidelines [Citation9,Citation10]. This may reflect the lack of a broadly accepted and widely used severity classification system for SSTIs and controversies regarding criteria for hospitalisation and the use of broad-spectrum antibiotics [Citation7,Citation11,Citation12]. The IDSA guidelines and the UK CREST guidelines both give recommendations based on severity, but at the same time these documents lack clearly defined severity criteria [Citation13,Citation14]. Marwick et al. developed the Dundee classification by modifying the CREST score, incorporating operational criteria using early warning scores for systemic severity [Citation7]. Recently, a large retrospective study suggested high reliability of the Dundee classification in predicting outcomes in cellulitis [Citation15]. Studies in this field are scarce, however. They have mainly been retrospective and included either exclusively cellulitis cases or a combination of purulent and non-purulent SSTIs, mitigating their clinical applicability.
There is a need for more specific studies on subsets of SSTIs, in order to provide appropriate severity scores, applicable in the clinical setting, which in turn could serve as a bridge between clinical management and antimicrobial guidelines [Citation15].
In this study, we used a prospectively enrolled cohort to describe the entire spectrum of severity in patients hospitalised with non-purulent SSTIs, ranging from cellulitis to NSTIs. We modified the Dundee classification by including relevant, novel tools for risk assessment in sepsis and evaluated the ability of the modified classification in predicting resource use and clinical outcome. Furthermore, we used the severity score to evaluate appropriateness of antimicrobial therapy in relation to severity and to identify risk factors and adverse effects associated with broad-spectrum antimicrobial therapy.
Materials and methods
Study population and ethics
The study was conducted at Haukeland University Hospital (HUH), a tertiary care hospital in western Norway, with a primary catchment area of approximately 300,000 inhabitants. Prospective inclusion was performed during 29 non-consecutive months, from September 2010 until August 2014. All patients included were aged 18 years or older and hospitalised with community acquired non-purulent SSTI in the form of cellulitis (including erysipelas) or NSTI. Four NSTI cases were transferred from nearby hospitals, shortly after arriving the respective emergency departments. The cellulitis patients correspond to the non-purulent part of a cohort previously analysed for aetiology and treatment response [Citation16,Citation17]. Cellulitis was defined as acute diffuse erythema of the skin of presumed bacterial origin and either (a) Fever, chills, or general illness, or (b) Facial infection. NSTI was defined as described by Calandra et al. [Citation18]. Patients with signs of purulent lesions were excluded, including patients with abscesses or those with history of recent purulence before admission, e.g. furuncles/folliculitis. Informed and written consent was obtained from all patients included. The study was approved by the Regional Ethics Committee (REC West; approval number 2010/1406).
Clinical characteristics, bacterial aetiology and outcome
For all cases, we completed a report form that included risk factors, clinical findings on admission (within 24 h), treatment, allergy towards antibiotics, nosocomial infections and adverse events e.g. diarrhoea (≥4 loose stools per day). Infectious disease specialists reviewed the clinical data entries and established the clinical SSTI diagnosis. The procedure for bacteriological and serological sampling and the criteria for probable and definite β–haemolytic streptococcal aetiology have been described previously [Citation16]. To evaluate the ability of the severity classification to predict the risk of complications and the appropriate level of care, we defined a complicated course as follows: Bacteraemia (blood culture positive for microbe not considered a contaminant); surgical escalation (the first surgery was performed >24 h after start of treatment, or a later surgery was more extensive than the preceding); admission to Intensive Care Unit (ICU) or high dependency unit (a medical ward with surveillance facilities offering intra-arterial blood pressure monitoring, vasopressor treatment and non-invasive ventilation support); or all-cause mortality within 30 days. Relapse was defined as an increase in symptoms or a new course of antibiotics between end of therapy (EOT) and two weeks after EOT or readmission with SSTI within 30 days of discharge. Relapse was assessed by telephone contact scheduled approximately two weeks after cessation of antimicrobial therapy.
Severity classification
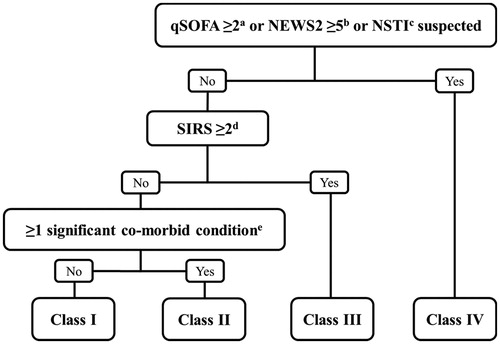
The severity score at admission was calculated retrospectively using a modified version of the Dundee score [Citation7]. We incorporated the quick Sequential Organ Failure Assessment (qSOFA) score developed as a risk assessment tool in accordance with the contemporary sepsis definition [Citation19]. Systemic inflammatory response syndrome (SIRS) criteria were also registered, as they are well-known markers of systemic response, to increase sensitivity in detecting patients with increased risk of severe outcomes [Citation7,Citation20]. Early warning score parameters were registered. We used the latest update of National Early Warning Score (NEWS2), as it has been shown to predict death and ICU transfer more accurately than other scoring systems in non-ICU patients [Citation21,Citation22]. A flow chart of the severity classification is shown in . We used the same co-morbid conditions as proposed elsewhere [Citation7,Citation14].
Figure 1. The modified Dundee classification. aqSOFA: quick Sequential Organ Failure Assessment score. bNEWS2: National Early Warning Score 2. Total NEWS ≥5 or 3 points in one variable. cNSTI: Necrotising Soft Tissue Infection. dSIRS: Systemic Inflammatory Response Syndrome. eSignificant co-morbid conditions: Peripheral vascular disease, chronic venous insufficiency, morbid obesity (BMI ≥40).
Antimicrobial therapy and appropriateness
All antibiotics given before and during the hospital stay, as well as after discharge, were recorded. ‘Broad-spectrum antimicrobial therapy’ was defined as gram-negative coverage for at least 24 h, while treatment with clindamycin or linezolid was regarded as use of efficient toxin inhibitor [Citation12]. Treatment escalation was defined as an addition of antimicrobial agent(s) or replacement with an antibiotic with broader antimicrobial spectrum, and de-escalation as the opposite. We used the severity classification described above to evaluate appropriateness of antimicrobial therapy (under-, over- or appropriate treatment). Appropriateness by severity class was assessed using our local recommendations for non-purulent SSTIs (), the IDSA guidelines [Citation13] and the CREST recommendations [Citation14]. The latter two recommendations are summarised in Supplementary Tables 1 and 2. Only the first antimicrobial regime initiated after admission was evaluated. In cases where the specific prescribed antimicrobials did not feature in the guidelines, assessment of appropriateness according to these guidelines was based on a consensus between the investigators (ER and TB).
Table 1. Local antimicrobial treatment recommendations for non-purulent SSTIs.
Statistics
Data were analysed using IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY). Categorical data were analysed using χ2 test or Fisher’s exact test. For continuous data, median and interquartile range or range was calculated, and Mann–Whitney U test or Kruskal–Wallis test was used as appropriate. All statistical tests were two-tailed, with p-values <.05 considered significant. To assess independent predictors of broad-spectrum antimicrobial treatment, diarrhoea, and prolonged treatment (>14 days of antimicrobial treatment), multivariate logistic regression analyses were performed. Predictors were chosen before fitting the models, based on previous studies and clinical experience. To estimate the accuracy of the severity score in predicting a complicated course, sensitivity, specificity, positive and negative likelihood ratios (LR+/LR−) were calculated.
Results
Clinical characteristics and severity
A total of 219 patients were included. Cellulitis and erysipelas accounted for 91% of the cases, and 9% were NSTIs (). The median age was 55 years, and 58% were male. Almost ¾ of the cases affected the limbs. By use of our modified Dundee severity score, 32% were categorised into severity class I, 15% in class II, 28% in class III and 25% in class IV. In comparison, 15% (32/219) were categorised into class IV when applying the original Dundee classification on this cohort [Citation7]. Among the 19 cases with a final diagnosis of NSTI, 18 fulfilled class IV criteria at admission. NSTI was suspected in eight of these 18 (44%) cases upon admission. Pain measured by Numerical Rating Scale (scale 0–10) was significantly more severe in class IV compared to class I–III patients (p = .007). In a post-hoc analysis, comparing NSTI and cellulitis, there were significantly more severe pain in the NSTI cohort (median 7 vs 2, interquartile range 6–8 vs 1–6, p < .001). Leucocyte count and procalcitonin concentration, but not C-reactive protein (CRP), were both positively associated to severity class IV (p < .001). Definite or probable β-haemolytic streptococcal aetiology was found in the great majority of the cases. Blood cultures were positive in 12 cases, the majority (10/12) among class IV patients, and 83% (10/12) showed β-haemolytic Streptococci.
Table 2. Demographics and clinical characteristics by severity classTable Footnotea.
Severity score and outcomes
presents outcomes in relation to severity class. Nosocomial infections were mainly observed in class IV, but relapse was not associated to severity class. The three fatal cases all belonged to severity class IV at admission and suffered from NSTIs, implying a 16% (3/19) mortality rate for the NSTI group. Among 60 cases fulfilling the defined criteria for having a complicated course, 41 cases had cellulitis. Among these, 85% (35/41) received treatment at ICU or high dependency unit care level.
Table 3. Treatment and outcome by severity classTable Footnotea.
Categorising patients into severity class III or IV implied a sensitivity of 83% and a specificity of 59% in predicting a complicated course. Using the original Dundee score on this cohort gave in comparison a sensitivity of 75% and specificity of 60%. The corresponding LR + for the two severity classifications were 2.01 and 1.86 respectively. The LR − was 0.29 in our severity score, versus 0.42 when using the Dundee score. With respect to the identification of NSTIs, class IV had a sensitivity of 95%, compared to a sensitivity of 47% using the original Dundee score. Being categorised into class I showed a very low LR − of 0.12 with respect to having a complicated course. In addition, having a complicated course was less frequent in head and neck infections compared to extremity infections (p = .002) and was associated with more severe pain (p < .001), but not with total body surface area (TBSA%) affected.
Antimicrobial treatment and appropriateness
Penicillin monotherapy as the initial antimicrobial therapy was given in 46% (100/219) of cases. Thirteen per cent (29/219) received other narrow-spectrum therapy (cloxacillin, clindamycin or cloxacillin plus penicillin), whereas combinations of clindamycin with penicillin or cloxacillin were initiated in 21% (47/219). Altogether 23 different antimicrobial regimens were initiated at admission. Twenty per cent (43/219) received gram-negative coverage initially, increasing to 27% (58/219) for the entire treatment course. Both clinical severity parameters and culture results were associated to gram-negative coverage, but only NEWS was found to be an independent predictor of such treatment [Odds Ratio (OR), 1.369; 95% confidence interval (CI), 1.173–1.597; p < .001, see Supplementary Table 3]. At discharge 26% (56/219) received clindamycin as part of the treatment, and 5% (12) received broader spectrum coverage.
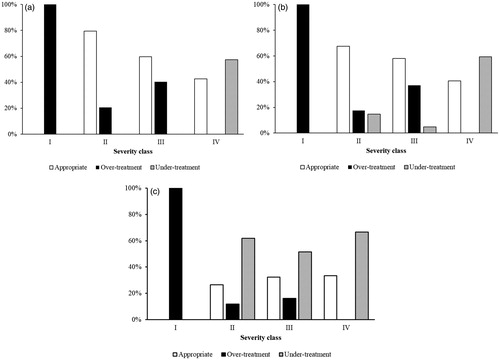
Antimicrobial therapy appropriateness assessed in relation to severity and different guidelines is presented in . In general, over-treatment was most common in the lower severity classes and under-treatment was most commonly observed in the higher severity classes. According to local recommendations, IDSA and CREST guidelines, over-treatment was identified in 46%, 45% and 38% of the cases, respectively. If narrow-spectrum intravenous therapy with penicillin and/or cloxacillin to class I patients was not deemed over-treatment, in accordance with common practice in Norway, the rates of over-treatment were reduced to 21%, 20% and 15%, respectively. Under-treatment was seen in 14%, 18% and 41% according to the three sets of recommendations. The high percentage of under-treatment according to CREST guidelines was mainly due to our use of penicillin treatment instead of the CREST recommended use of flucloxacillin/cloxacillin. A post-hoc analysis of appropriateness of therapy in relation to a complicated course showed significantly more under-treatment in those having a complicated course (p = .001).
Figure 2. Treatment appropriateness according to severity class. (a) Treatment appropriateness according to local treatment recommendations. (b) Treatment appropriateness according to IDSA guidelines. (c) Treatment appropriateness according to CREST guidelines.
In a multivariate analysis, receiving broad-spectrum antibiotics or clindamycin during the course was significantly associated with diarrhoea (OR, 3.789; 95%CI, 1.611–8.908; p = .002), which was reported in 20% of cases during or after their stay (Supplementary Table 4). Prolonged treatment duration (>14days) was associated to age (OR 1.054; 95%CI, 1.024–1.084; p < .001), surgery (OR 27.901; 95%CI 8.664–89.854; p < .001) and increasing NEWS (OR 1.173; 95%CI, 1.011–1.361; p = .035) (Supplementary Table 5).
Discussion
In this study, we have analysed severity scores, appropriateness of antimicrobial therapy and patient outcomes in an unselected prospective cohort of non-purulent SSTIs admitted for in-hospital antibiotic treatment. We found that our revised Dundee scoring system demonstrated a high clinical applicability; useful for rapidly identifying patients in need of urgent intervention (such as NSTIs), as well as sorting out uncomplicated cellulitis cases suitable for outpatient treatment.
The study includes both cases with cellulitis and NSTI, clinical entities that are often hard to discern in the early phase [Citation6], reflecting the diagnostic challenges seen in every-day handling of non-purulent SSTIs. Cellulitis constituted the majority of cases, and almost half of the patients belonged to severity classes I or II, suggesting that many patients are admitted for reasons other than systemic toxicity. A significant proportion of these patients had complicating factors, including diabetes, immunosuppression and other relevant comorbidities, probably contributing to hospitalisation.
In this unselected population as many as one in four of the patients were in class IV, most of them admitted to the ICU or a high dependency unit. This clearly demonstrates that even though septic shock and bacteraemia is infrequent in cellulitis, non-purulent SSTIs requires significant health care resources, not only due to a high burden of disease, but also because of severity or suspected severe disease, as previously shown [Citation6]. In comparison, using the original Dundee classification, fewer cases were categorised into severity class IV (25% vs 15%). In the two original papers by Marwick et al. 6% and 14%, respectively, were categorised into class IV [Citation7,Citation23]. In the first study, patients were retrospectively included, and eligible patients comprised a wide range of different SSTI codes [Citation7]. The second study included prospectively enrolled cellulitis, erysipelas or NSTI patients (n = 79), and demonstrated a comparable 14% of cases fulfilling class IV criteria [Citation23]. Both the herein used NEWS instead of Standard Early Warning Score (SEWS) and the additional criterion of including ‘NSTI suspected’ for class IV severity is likely the reason why our study had a higher proportion in class IV. These findings support the validity of the original Dundee score for non-purulent SSTIs. In addition, our modification is in line with increasing evidence of NEWS to be the preferred early warning score, also in the emergency department [Citation21,Citation22].
In this study, all patients with a qSOFA score ≥2 also had a NEWS ≥5, suggesting that the qSOFA criterion in our severity score could be redundant. It is possible, however, to have a qSOFA score of 2 and a NEWS of 4. Moreover, qSOFA is a score commonly in use. Instead of using NEWS only and setting the cut off to 4, we included qSOFA to increase sensitivity without reducing specificity significantly.
Ruling out NSTI is probably the most crucial and difficult task when facing severe non-purulent SSTIs. Our modified Dundee severity score correctly categorised 18 of 19 cases that turned out to be necrotising infections into class IV. One might argue that having the criterion ‘NSTI suspected’ to classify patients into class IV is non-valid. However, in detecting NSTIs clinical suspicion seems to be better than current laboratory testing or use of the LRINEC (Laboratory Risk Indicator for Necrotising Fasciitis) score [Citation8]. We found pain to be significantly more severe in NSTI cases, confirming that clinical pain assessment is important in the clinical evaluation of possible NSTIs [Citation8]. The composite outcome, ‘complicated course’ was predicted with a sensitivity of more than 80% for severity class III or IV, supporting the usefulness of the score in predicting resource use and outcome. Our classification was slightly more sensitive and demonstrated a higher LR + than the original Dundee score. Our severity score demonstrated a low LR − value with respect to having a complicated course, indirectly supporting its use in evaluation of possible NSTIs. In class I patients, the frequency of a complicated course was very low and clearly different from the other classes, as shown with a very low LR−. This supports the differentiation of class I from class II when making decisions on which patients that need to be hospitalised.
Expectedly, we found that not all cases with a complicated course were in the highest severity classes, as the score system is a simple tool including mainly easily accessible clinical parameters. This underscores the need for considering also additional factors when deciding management. The IDSA guidelines propose a list of conditions that should prompt consideration of hospital admission and systemic antibiotics, including malignancy on chemotherapy, neutropenia, severe cell-mediated immunodeficiency, clinical signs of deep infections, outpatient treatment failure and poor adherence to therapy. Other authors also include additional factors like facial or hand location and lesion size [Citation24], but neither facial location nor lesions size were among the characteristics that were associated to a complicated course in our study. Lack of predictive power of severity assessments at admission may also be due to deterioration later in the course. Marwick et al. demonstrated that vital parameters often worsen during the first 24 h [Citation23]. We found that some class I and II patients had a complicated course. Re-evaluation of severity also after admission as well as proper information to patients not admitted is therefore essential.
As could be expected, treatment was more appropriate when evaluated against our local recommendations, compared to evaluation against CREST and IDSA guidelines.
Of concern, over-treatment was common regardless of guideline used for evaluation, and mainly associated with the lower severity classes, which constitute the great majority of patients. This has also been demonstrated in previous studies [Citation7,Citation23,Citation25]. In most cases, over-treatment was due to treatment by the intravenous route instead of oral administration. In Norway, narrow-spectrum intravenous therapy with penicillin or cloxacillin administered to class I patients is commonly practiced, particularly in infections of the head or when treating extensive lesions [Citation16,Citation26], without other severe signs. Similar practice has also been reported outside the Scandinavian countries [Citation27]. To consider such therapy as over-treatment is debateable in our setting, although increasing knowledge supports use of oral antimicrobial therapy or outpatient intravenous therapy in such cases [Citation28,Citation29]. A disadvantage of early outpatient therapy is that it frequently involves more broad-spectrum drugs.
Frequent broad-spectrum antimicrobial therapy in cases without predisposing conditions associated to gram-negative infections is noteworthy. This could partly be caused by culture results of dubious significance, although culture of gram-negatives was not an independent factor associated with broad-spectrum therapy. Such practice has been detected by other studies [Citation30,Citation31], and deviates from guidelines and recent knowledge on the aetiology of cellulitis [Citation13,Citation16,Citation32]. Use of broad-spectrum antimicrobial therapy or clindamycin was associated with diarrhoea in our study, which is in line with other data linking clindamycin use in cellulitis and diarrhoea [Citation33]. Unrestricted clindamycin use is unwanted also due to increasing rates of resistance seen in e.g. Streptococcus dysgalactiae [Citation34], an emerging microbial cause of cellulitis [Citation35–37]. The possible benefit of clindamycin is primarily shown in severe infections [Citation38]. A recent study confirmed that clindamycin has no role as an adjunctive in uncomplicated cellulitis [Citation33]. Therefore, the high rates of clindamycin use found in our and other studies, is of concern, and measures to reduce this overuse of clindamycin should be sought [Citation5,Citation27].
Prolonged treatment was common, as confirmed elsewhere, and associated with increasing age and severity [Citation15,Citation39]. Randomised trials involving subgroups that may need prolonged treatment are warranted to evaluate what risk groups that truly benefit from prolonged therapy.
Under-treatment was most commonly observed in the higher severity classes, in line with previous data [Citation7,Citation25]. This is particularly worrisome, as it may reflect a delay in discerning the severity in a group of patients that not only needs broad-spectrum antimicrobial therapy, but frequently also a prompt and multidisciplinary approach that may include surgery. Because of the low mortality rate in our cohort, the effect of antibiotic appropriateness on mortality was not possible to evaluate, however, but we did find that under-treatment was associated with having a complicated course.
A strikingly high number of different antimicrobial regimes were initiated upon admission, although not as many as detected by Marwick et al. [Citation7], again underlining the need for increased adherence to guidelines [Citation9,Citation10]. Furthermore, blood cultures were taken in the vast majority (95%), yet positive cultures were exclusively detected in cases in severity class III or IV. This is in conformity with very low rates of bacteraemia reported in studies of uncomplicated cellulitis, and supports a more restricted use of blood cultures [Citation12,Citation32,Citation35]. In accordance with this, the IDSA guidelines recommend blood cultures in patients with active malignancy, on chemotherapy, neutropenia, severe cell-mediated immunodeficiency, immersion injuries and animal bites [Citation13]. Being categorised into severity class III or IV could also be added to this list, in line with the CREST guidelines and the data in our study [Citation14].
To move forward in improving clinical management of SSTIs, and non-purulent SSTIs in particular, the systematic use of severity scores may be one important measure, preferentially implemented as part of facility-specific care pathways or guidelines, as has been done in Auckland, New Zealand [Citation15]. Standardised care for cellulitis and other SSTIs has been shown to reduce use of both broad-spectrum antibiotics and hospital costs [Citation10,Citation40,Citation41].
Major strengths of this study include the prospective design and the limited number of experienced clinicians involved in inclusion and evaluation of patients. Furthermore, including only a well-defined population of non-purulent SSTIs made it easier to evaluate therapy against different guidelines. In studies of SSTIs in general, the considerable heterogeneity also reduces the ability to identify factors associated to treatment and severe/adverse outcomes. We applied a scorings system on the entire spectrum of severity, including also necrotising infections. This increases the clinical relevance, since necrotising and non-necrotising cases do not present as clear-cut categories. The major limitation of the study relates to the low number of mortalities, as was expected in a moderate-size study of a condition that rarely leads to death [Citation28]. Therefore, a composite outcome measure was incorporated to validate the score. The low prevalence of MRSA in our region should not affect the applicability of the findings, as recent study results indicate that MRSA does not have a major role in non-purulent SSTIs [Citation32,Citation42,Citation43].
Conclusions
In conclusion, a simple severity scoring system incorporating recently developed assessment tools proved valuable in identifying those with the highest and lowest risk (i.e. class IV and class I cases), and could serve as an useful clinical tool in the emergency department. Almost one third of the patients belonged to the lowest severity class, suggesting more patients might be treated as outpatients. Substantial overuse of broad-spectrum antimicrobial regimes, including frequent adjunctive clindamycin use, was observed, and was associated with diarrhoea. Several factors associated to prolonged treatment were identified. More systematic use of severity assessment tools could potentially have a significant impact on the appropriateness of therapy and level of care, in addition to serve as standardised criteria for severity in clinical trials.
Author contributions
E.R designed the study, included cases and collected data, performed the data analyses and drafted the manuscript. T.B. designed the study, included cases, collected data and drafted the manuscript. S.S. participated in the design of the study, inclusion of cases, collection of data, and drafting the manuscript. O.O. participated in inclusion of cases, collection of data, and drafting the manuscript. All authors approved the submitted version
Supplemental Material
Download MS Word (21.4 KB)Acknowledgements
The authors thank co-workers at Haukeland University Hospital who have contributed to the study.
The study was presented in part at the 28th European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain, 21–24 April 2018.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ansari F, Erntell M, Goossens H, et al. The European surveillance of antimicrobial consumption (ESAC) point-prevalence survey of antibacterial use in 20 European hospitals in 2006. Clin Infect Dis. 2009;49:1496–1504.
- Christensen KL, Holman RC, Steiner CA, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025–1035.
- Cannon J, Rajakaruna G, Dyer J, et al. Severe lower limb cellulitis: defining the epidemiology and risk factors for primary episodes in a population-based case-control study. Clin Microbiol Infect. 2018;24:1089–1094.
- Tun K, Shurko JF, Ryan L, et al. Age-based health and economic burden of skin and soft tissue infections in the United States, 2000 and 2012. PLoS One. 2018;13:e0206893.
- Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325–337.
- Cranendonk DR, van Vught LA, Wiewel MA, et al. Clinical characteristics and outcomes of patients with cellulitis requiring intensive care. JAMA Dermatol. 2017;153:578–582.
- Marwick C, Broomhall J, McCowan C, et al. Severity assessment of skin and soft tissue infections: cohort study of management and outcomes for hospitalized patients. J Antimicrob Chemother. 2011;66:387–397.
- Goh T, Goh LG, Ang CH, et al. Early diagnosis of necrotizing fasciitis. Br J Surg. 2014;101:e119–25.
- Kamath RS, Sudhakar D, Gardner JG, et al. Guidelines vs actual management of skin and soft tissue infections in the emergency department. Open Forum Infect Dis. 2018;5:ofx188.
- Nymoen Aasbrenn M, Skeie I, Berild D. Compliance to antibiotic guidelines leads to more appropriate use of antibiotics in skin and soft tissue infections in injecting drug users. Infect Dis (Lond). 2019;51:570–577.
- Claeys KC, Zasowski EJ, Lagnf AM, et al. Development of a risk-scoring tool to determine appropriate level of care in acute bacterial skin and skin structure infections in an acute healthcare setting. Infect Dis Ther. 2018;7:495–507.
- Montravers P, Snauwaert A, Welsch C. Current guidelines and recommendations for the management of skin and soft tissue infections. Curr Opin Infect Dis. 2016;29:131–138.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10–52.
- Clinical Resource Efficiency Support Team (CREST). Guidelines on the Management of Cellulitis 2005 [cited 2018 Apr 30]. Available from: http://www.rcem.ac.uk/docs/External%20Guidance/10n.%20Guidelines%20on%20the%20management%20of%20cellulitis%20in%20adults%20(CREST,%202005.pdf
- Cutfield T, Walter H, Hobbs M, et al. Association of the Dundee severity classification with mortality, length of stay and readmission in adult inpatients with cellulitis. J Antimicrob Chemother. 2019;74:200–206.
- Bruun T, Oppegaard O, Kittang BR, et al. Etiology of cellulitis and clinical prediction of streptococcal disease: a prospective study. Open Forum Infect Dis. 2016;3:ofv181.
- Bruun T, Oppegaard O, Hufthammer KO, et al. Early response in cellulitis: a prospective study of dynamics and predictors. Clin Infect Dis. 2016;63:1034–1041.
- Calandra T, Cohen J. International sepsis forum definition of infection in the ICUCC. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33:1538–1548.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–810.
- Maitra S, Som A, Bhattacharjee S. Accuracy of quick sequential organ failure assessment (qSOFA) score and systemic inflammatory response syndrome (SIRS) criteria for predicting mortality in hospitalized patients with suspected infection: a meta-analysis of observational studies. Clin Microbiol Infect. 2018;24:1123–1129.
- Churpek MM, Snyder A, Han X, et al. Quick sepsis-related organ failure assessment, systemic inflammatory response syndrome, and early warning scores for detecting clinical deterioration in infected patients outside the intensive care unit. Am J Respir Crit Care Med. 2017;195:906–911.
- Silcock DJ, Corfield AR, Rooney KD, et al. Superior performance of national early warning score compared with quick sepsis-related organ failure assessment score in predicting adverse outcomes: a retrospective observational study of patients in the prehospital setting. Eur J Emerg Med. 2019;26:433–439.
- Marwick C, Rae N, Irvine N, et al. Prospective study of severity assessment and management of acute medical admissions with skin and soft tissue infection. J Antimicrob Chemother. 2012;67:1016–1019.
- Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: a review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol. 2008;19:173–184.
- Hashem NG, Hidayat L, Berkowitz L, et al. Management of skin and soft-tissue infections at a community teaching hospital using a severity-of-illness tool. J Antimicrob Chemother. 2016;71:3268–3275.
- Rath E, Skrede S, Mylvaganam H, et al. Aetiology and clinical features of facial cellulitis: a prospective study. Infect Dis (Lond). 2018;50:27–34.
- Kiely A, Elwahab SA, McDonnell D, et al. Over-admission and over-treatment of patients with cellulitis: a 5-year audit against international guidelines. Ir J Med Sci. 2020;189:245–249.
- Gunderson CG, Cherry BM, Fisher A. Do patients with cellulitis need to be hospitalized? Asystematic review and meta-analysis of mortality rates of inpatients with cellulitis. J Gen Intern Med. 2018;33:1553–1560.
- Ong BS, Ngian VJJ, Yeong C, et al. Out of hospital and in hospital management of cellulitis requiring intravenous therapy. Int J Gen Med. 2019;12:447–453.
- Garau J, Ostermann H, Medina J, et al. Current management of patients hospitalized with complicated skin and soft tissue infections across Europe (2010-2011): assessment of clinical practice patterns and real-life effectiveness of antibiotics from the REACH study. Clin Microbiol Infect. 2013;19:E377–E385.
- Jenkins TC, Knepper BC, Moore SJ, et al. Antibiotic prescribing practices in a multicenter cohort of patients hospitalized for acute bacterial skin and skin structure infection. Infect Control Hosp Epidemiol. 2014;35:1241–1250.
- Jeng A, Beheshti M, Li J, et al. The role of beta-hemolytic streptococci in causing diffuse, nonculturable cellulitis: a prospective investigation. Medicine (Baltimore). 2010;89:217–226.
- Brindle R, Williams OM, Davies P, et al. Adjunctive clindamycin for cellulitis: a clinical trial comparing flucloxacillin with or without clindamycin for the treatment of limb cellulitis. BMJ Open. 2017;7:e013260.
- Laboratory surveillance of pyogenic and non-pyogenic streptococcal bacteraemia in England, Wales and Northern Ireland: 2016. England: PHE publication; 2017 [cited 2019 May 30]. Available from: www.gov.uk/phe; https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/660589/hpr4117_pnp-strptccc.pdf
- Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64:148–155.
- Siljander T, Karppelin M, Vahakuopus S, et al. Acute bacterial, nonnecrotizing cellulitis in Finland: microbiological findings. Clin Infect Dis. 2008;46:855–861.
- Oppegaard O, Mylvaganam H, Kittang BR. Beta-haemolytic group A, C and G streptococcal infections in Western Norway: a 15-year retrospective survey. Clin Microbiol Infect. 2015;21:171–178.
- Carapetis JR, Jacoby P, Carville K, et al. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infections. Clin Infect Dis. 2014;59:358–365.
- Figtree M, Konecny P, Jennings Z, et al. Risk stratification and outcome of cellulitis admitted to hospital. J Infect. 2010;60:431–439.
- Yarbrough PM, Kukhareva PV, Spivak ES, et al. Evidence-based care pathway for cellulitis improves process, clinical, and cost outcomes. J Hosp Med. 2015;10:780–786.
- Housman E, Livings SE, Knee A, et al. Improving management of hospitalized adults with uncomplicated cellulitis or cutaneous abscess. Open Forum Infect Dis. 2017;4:ofx094.
- Pallin DJ, Binder WD, Allen MB, et al. Clinical trial: comparative effectiveness of cephalexin plus trimethoprim-sulfamethoxazole versus cephalexin alone for treatment of uncomplicated cellulitis: a randomized controlled trial. Clin Infect Dis. 2013;56:1754–1762.
- Moran GJ, Krishnadasan A, Mower WR, et al. Effect of cephalexin plus trimethoprim-sulfamethoxazole vs cephalexin alone on clinical cure of uncomplicated cellulitis: a randomized clinical trial. JAMA. 2017;317:2088–2096.
