Abstract
Background
The long-term sequelae after COVID-19 are not yet fully known. Our aim was to evaluate subjective symptoms and quality of life in Finnish hospitalized COVID-19 patients at six months follow-up.
Methods
Hospitalised adult patients with laboratory-confirmed SARS-CoV-2 infection from March to June 2020 were recruited. We conducted a survey on demographics and comorbidities, ten specific symptoms, and a RAND-36 quality of life questionnaire six months after hospital discharge. We collected clinical data manually from medical records.
Results
101 patients (54 male) out of 246 invited completed the survey. Their median age was 60 years, and the mean hospital length of stay was 15 d. Most patients (90%) experienced symptoms, the most common of which were tiredness (88%), fatigue (79%), sleeping problems (76%), and dyspnoea (70%). In regard to gender, women showed a shorter time of hospitalization (p = .048) and lower peak flow of supplementary oxygen (p = .043). Women reported more frequently dyspnoea, fatigue, tiredness, sleeping problems, and mood problems (p = .008–.033), and a lower quality of life in seven of eight dimensions (p < .001–.015). Five explanatory variables for the reduced quality of life were identified in multivariate analysis: age, female sex, BMI, sleep apnoea, and duration of mechanical ventilation. Of the patients who worked full-time before COVID-19, 11% had not returned to work.
Conclusions
Most patients experienced symptoms six months after hospital discharge. Women reported more symptoms and a lower quality of life than men. These findings highlight the differences in recovery between men and women and call for active rehabilitation of COVID-19 patients.
Introduction
In the Helsinki-Uusimaa hospital district in Finland, the first Coronavirus disease 2019 (COVID-19) cases were diagnosed on 20 February 2020. By the end of February 2021, more than 56 000 cases of COVID-19 had been reported in Finland (population 5.6 million) and more than 13 000 in southern Finland in the region of this study (population 1.7 million) [Citation1,Citation2].
World Health Organization has reminded us that many patients suffer from long-term symptoms after COVID-19 [Citation3]. Studies from the origin of infection, Wuhan, China, report that almost half of the hospitalized patients had general symptoms with respiratory problems most common still three months after hospital discharge [Citation4]. Six months after symptom onset, almost two-thirds of the patients complained of fatigue and one-fourth of sleeping problems [Citation5]. Exercise capacity was below the lower limit of normal in one-quarter of these patients irrespective of the severity of the disease. This reminds me of the earlier human coronavirus infection Severe acute respiratory syndrome (SARS), where an impaired exercise capacity as well as a decreased quality of life, have been described after 6 months and up to two years after initial infection [Citation6–Citation8].
Accordingly, the first studies have shown persisting symptoms as well as lung function impairment and radiological abnormalities [Citation5,Citation9]. Knowledge of persisting symptoms and sequelae should be well described for the best rehabilitation of the patients. An international task force of pulmonary rehabilitation experts recommends assessing emotional and physical recovery at six to eight weeks after hospital discharge, and pulmonary rehabilitation or muscle strengthening, if lung function or muscle impairment is present, respectively [Citation10]. A similar follow-up schedule is recommended by the British Thoracic Society and the British Society of Thoracic Imaging [Citation11].
Our aim was to evaluate subjective long-term symptoms and their effect on the quality of life in Finnish COVID-19 survivors and the association of these with the individual patient-based and in-hospital factors.
Materials and methods
Patient selection
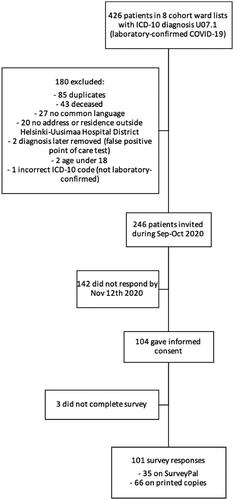
Hospitalized patients with a laboratory-confirmed COVID-19 treated in COVID-19 cohort wards in Helsinki University Hospital, between 1 March and 24 June were invited to participate in the study (). Patients who were aged 17 years or under, or who lacked a common language with the research team (Finnish, Swedish, or English), were excluded from the study. Patients were identified from the patient records with international classification of diseases code (ICD-10) U07.1 (laboratory-confirmed COVID-19 infection) among patients treated in cohort wards in Helsinki. Patients were invited to participate in the study during September and October 2020.
Methods
Participants filled an electronic survey on SurveyPal or a printed copy. The survey consisted of 12 questions about background information (age, gender, height and weight, previously diagnosed conditions, nicotine product use, employment status, and duration of sick leave), symptoms, the modified Medical Research Council dyspnoea scale (mMRC), and a 36-item quality of life questionnaire. The survey was available in three languages (Finnish, Swedish, and English). The survey was completed on average in 174 d (median 180 d) after hospital discharge. The electronic survey was filled by 35 patients (34.7%) and the printed version by 66 patients (65.3%), respectively.
Experienced symptoms were inquired about one month before the COVID-19 infection and at the time of answering the questionnaire. Symptoms were reported in ten different aspects, which were cough, dyspnoea, fatigue, tiredness, dizziness, gastrointestinal symptoms, difficulties while moving, sleeping problems, loss of taste or smell, and mood problems. Participants reported symptoms on a scale of 0 to 10, with 0 being ‘not at all’ and 10 being ‘all the time’.
Quality of life was assessed using the RAND-36 questionnaire, which measures the quality of life in eight scales: physical functioning, physical role functioning, emotional role functioning, energy/fatigue, emotional well-being, social functioning, bodily pain, and general health perceptions [Citation12]. The questionnaire was scored using the RAND-36 scoring instructions [Citation13].
Clinical data of initial infection were obtained manually from electronic medical records, including durations of hospital stay, intensive care, and mechanical ventilation, peak flow of supplementary oxygen, treatment, thrombotic complications, and laboratory test results (C-reactive protein (CRP), white blood cell count, lymphocyte count, alanine transaminase, and D-dimer). Reported comorbidities and other demographic factors were cross-checked with medical records and supplemented where necessary.
Statistical analysis
Between-group differences were analysed using the Mann-Whitney-U test, and repeated-measure differences using the Wilcoxon signed rank-sum test. The significance level was set at 0.05.
A structural equation model was used to model RAND-36 dimensions dependency on the patient-based and in-hospital variables. RAND-36 dimensions were divided into physical (physical functioning, physical role functioning, bodily pain, and general health perceptions) and emotional dimensions (emotional role functioning, energy/fatigue, emotional well-being, and social functioning) and analyzed separately. Two latent factors, termed L1 and L2, were assumed to be behind these two groups of variables and used as the main dependent variables. All variables, which had at least 5% different values, did not have too many missing values and were considered as clinically relevant, were used as candidate explanatory variables in the model. Patient-based variables were used as exogenous variables, which could have either a direct effect on the latent factors or an indirect effect through the in-hospital variables. The explanatory variables in the final model were selected by backward selection using the Akaike information criterion or Bayesian information criterion.
Ethics
This study was a prospective clinical observational study and the study protocol was approved by the Research Ethics Committee of Helsinki University Hospital (§148 HUS/1922/2020). All aspects of the study were conducted within the principles of Good Clinical Practice and in accordance with the Declaration of Helsinki. All participants gave their written informed consent.
Results
Study population
Participants were 101 out of 246 invited patients (response rate 41%) hospitalized due to a laboratory-confirmed SARS-CoV-2 infection during spring and summer 2020 in cohort wards in Helsinki. Their median age was 60 years, ranging from 26 to 81 (). Of the patients, 54 (53%) were male. One or more comorbidities were found in 70 patients (69%), the three most common being hypertension (42, 42%), asthma (19, 19%), and hyperlipidaemia (19, 19%). Asthma was more common in women (p < .001). Rheumatoid arthritis was found only in women and neurological diseases only in men. There were no statistically significant differences in any other comorbidities between the sexes.
Table 1. Baseline characteristics of the study population.
The clinical characteristics of the study population are presented in . The mean duration of hospital treatment was 15 d. Women had a shorter length of stay than men (mean 12 vs. 18 d, p = .048). Of the patients, 34 (34%) were admitted to an intensive care unit (ICU) and 23 (23%) needed mechanical ventilation. The mean duration of ICU treatment and the mean duration of mechanical ventilation was 15 d and 16 d, respectively. Of the 78 non-ventilated patients, 58 (74%) needed supplementary oxygen therapy. The mean peak flow rate of supplementary oxygen was 8 l/min, ranging from 1 to 15 l/min. The mean peak flow rate of oxygen was higher in men than in women (7 vs. 10 l/min, p = .043). Women also had lower peak CRP values as compared to men (p = .017) whereas no other significant differences in laboratory values or medical treatment between the genders were noted ().
Table 2. Clinical characteristics of the 101 hospitalized COVID-19 patients that answered the questionnaire.
Sixty-three patients (62%) were working full-time before COVID-19, of whom 52 (83%) had returned to work full-time, four (6%) part-time, and seven (11%) had not returned to work six months after hospital discharge. Those who had not returned to work were 56–73 in age (median 57). Eight (8%) reported working part-time before COVID-19, three of whom had returned to work part-time, three gave no answer, and two had not returned to work.
Symptoms and medical research council dyspnoea scale (mMRC)
The symptoms and mMRC results are presented in and . The response rate in symptom questions ranged from 88 to 95%. Symptoms were classified as mild if the answer was 1–5 and severe if the answer was scaled 6–10.
Figure 2. Symptoms, mMRC, and quality of life at 6 monthsfollow-up among the 101 COVID-19 patients. a) Data are presented as percentages of responses. Response rate 93–98% in men and 89–94% in women. b) mMRC, modified Medical Research Council dyspnoea scale; grade 0, ‘dyspnoea only with strenuous exercise’; grade 1, ‘dyspnoea when hurrying or walking up a slight hill’; grade 2, ‘I walk slower than people of the same age because of dyspnoea or have to stop for breath when walking at own pace’; grade 3, ‘I have to stop for breath walking 100 meters or after walking a few minutes at my own pace on the level’; grade 4, ‘I am too breathless to leave the house, or breathless when dressing/undressing’. Data are presented as the number of responses (N). Response rate 91% in men and 94% in women. c) Data are presented as means of responses. RAND-36 questionnaire assesses the quality of life in eight dimensions on a scale of 0 to 100, 100 being the best score. Response rate 94–100% in men and 94–98% in women.
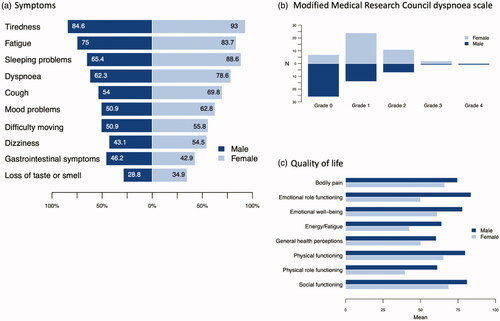
Table 3. Symptoms and mMRC at 6 months months follow-up among the 101 hospitalized COVID-19 patients.
Ninety-one patients (90% of the study population) reported one or more symptoms at six months. The most common symptoms were tiredness (84/95, 88%), fatigue (75/95, 79%), and sleeping problems (73/96, 76%). Dyspnoea was reported by 66 patients (70%) and cough by 57 (61%), respectively. The severity of symptoms was mild in 78% and severe in 22% of patients. However, 43 patients (45%) reported one or more severe symptoms, with seven patients (7%) reporting two, eight patients (8%) three, 11 (12%) four, and six (6%) reporting five or more severe symptoms. Only six patients (6% of the study population) reported no symptoms at six months, and four (4%) did not give an answer to symptoms at six months.
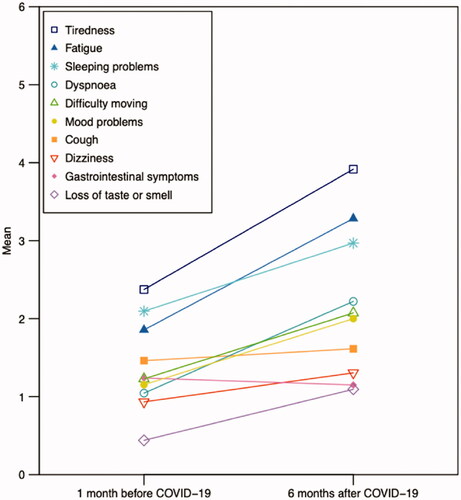
On average, patients reported more symptoms at six months compared to one month before COVID-19 in all symptom aspects, except gastrointestinal symptoms (). The differences were statistically significant in dyspnoea (p < .001), fatigue (p < .001), tiredness (p < .001), dizziness (p = .013), difficulty moving (p = .001), sleeping problems (p = .001), loss of taste or smell (p = .002), and mood problems (p < .001). Symptoms increased most in tiredness, fatigue, and dyspnoea, where an increase was found in 51 of 90 responses (57%), 45 of 89 (51%), and 40 of 87 (46%), respectively.
Figure 3. Reported symptoms one month before and six months after COVID-19 in the 101 hospitalized patients.
Women had more symptoms than men in every symptom aspect, except gastrointestinal symptoms. The differences were significant in dyspnoea (p = .024), fatigue (p = .033), tiredness (p = .012), sleeping problems (p = .008), and mood problems (p = .031). Additionally, women graded a greater disability due to dyspnoea as measured by the mMRC, with most men reporting grade 0 and most women reporting grade 1 (mean 0.71 vs. 1.18, p = .002).
Quality of life
Quality of life was assessed in eight dimensions on a scale of 0 to 100, 100 being the best score. The results are presented in and .
Table 4. Quality of life at 6 months months follow-up among 101 hospitalized COVID-19 patients assessed by RAND-36.
Women had lower scores than men in all eight dimensions of the RAND-36 questionnaire. The differences were statistically significant in physical functioning (p = .001), role limitations due to physical problems (p = .015), role limitations due to emotional problems (p < .001), energy/fatigue (p < .001), social functioning (p = .015), and general health conceptions (p = .005).
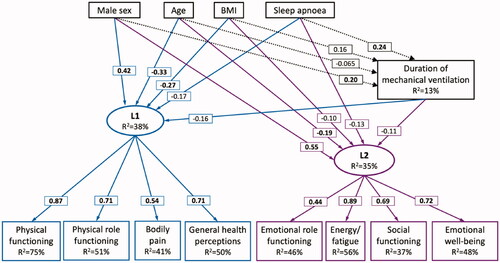
The structural equation model for the quality of life dimensions is illustrated in . Five explanatory variables for the decreased quality of life were identified: age, sex, BMI, obstructive sleep apnoea as a comorbidity, and duration of mechanical ventilation. Male sex had a positive effect on both physical and emotional dimensions of the quality of life. Age, BMI, sleep apnoea, and duration of mechanical ventilation were associated with lower scores in physical and emotional dimensions. Additionally, age was associated with a shorter duration of mechanical ventilation, while male sex, BMI, and sleep apnoea were associated with a longer duration.
Figure 4. Structural equation model for RAND-36 quality of life dimensions. Sex, age, BMI, sleep apnoea, and duration of mechanical ventilation are the explanatory variables. L1 and L2 are latent explanatory variables for physical (L1) and emotional (L2) dimensions of the RAND-36 quality of life. Coefficients are presented in boxes on the arrows, with coefficients with p < .05 in bold. R2 is the coefficient of determination.
Discussion
The main finding in this study was that 90% of patients reported suffering from some symptoms still six months after hospital treatment for COVID-19, and 11% of those who had worked full-time before COVID-19, had not returned to work. Interestingly, women reported more symptoms and more decreased quality of life in women as compared to men. This occurred despite the women had shorter hospital admissions, lower supplementary oxygen need and lower inflammatory markers during hospitalization.
Nearly half of the patients suffered from one or more symptoms defined as severe six months after the infection. Our finding is in line with a recent study from patients in Wuhan, that indicated that there were dysfunctions and complications among the COVID-19 patients after 6 months of follow-up [Citation4]. In our study, the most common symptoms after six months were tiredness, fatigue, sleep problems, and dyspnoea. This is in line with previous studies [Citation5,Citation9]. In addition, the cough was present in over half of the patients, which to our knowledge has not been reported in previous follow-up studies of COVID-19 patients. Together these studies point out that COVID-19 indeed has long-term effects that should be considered when caring for these patients.
Our finding is in line with previous reports on COVID-19 patients’ characteristics, regarding the age and sex distribution, and the high prevalence of overweight patients and patients with hypertension [Citation5,Citation9,Citation14,Citation15]. Moreover, we found no differences between the genders in age or BMI distribution. Interestingly, we observed that women had a milder disease as assessed by the shorter length of hospital stay, lower oxygen supplementation need and lower peak CRP level. Yet, women suffered more commonly from long-term symptoms than men. Women also graded a bigger decline in their quality of life than men. Huang et al. also reported women having more complaints but did not describe the differences [Citation5]. Answer to this gender difference may not be found in the background characteristics of the patients, since women only had more asthma than men without any other differences in the demographic factors. The more serious COVID-19 disease in men has been previously reported [Citation16,Citation17], but the poorer long-term recovery and longer persisting symptoms in women need more investigation.
Quality of life was evaluated with a standardized RAND-36 method, which includes the same items as the 36-item Short-Form Health Survey (SF-36) [Citation12]. To our knowledge, there are no previous studies published that have used RAND-36 or SF-36 to evaluate the quality of life after COVID-19 infection, even though there are numerous studies of previous SARS and the Middle East respiratory syndrome (MERS) survivors [Citation6,Citation7,Citation18]. According to our study, patients with COVID-19 appear to suffer from the same prolonged impairment in quality of life as was the case in SARS and MERS. In multivariate analysis, we found that lower quality of life was associated with female sex, greater age, higher BMI, sleep apnoea as a comorbidity, and a longer duration of mechanical ventilation. Our findings call for phenotyping COVID-19 patients and finding those patients that are prone to long-term symptoms and impaired quality of life, to actively rehabilitate especially these patients.
This study has some limitations. Less than half of the recruited patients participated in this study, which might lead to an increased prevalence of symptoms, as we hypothesize that patients with more or more difficult symptoms are more likely to participate. Recall bias is obvious when patients are reporting a change in symptoms in a non-prospective setting. In addition, we only included hospitalized and laboratory-confirmed patients in our study, and a considerable percentage of participants had required intensive care or mechanical ventilation. Therefore, these results only represent patients with a severe COVID-19 infection and cannot be directly applied to outpatients.
The ongoing pandemic itself can have negative effects on well-being, and quarantine has been shown to have negative effects on both quality of life and mental health [Citation19]. As our study did not have a control group, we cannot assess whether our findings of quality of life after COVID-19 are due to the infection itself or due to other factors. Recent studies have, however, shown the impaired diffusing capacity and decreased lung volume in COVID-19 survivors from hospital discharge to up to six months after the onset of symptoms. Impaired diffusing capacity can cause dyspnoea and fatigue [Citation20], and could explain these symptoms also in our study population.
Our study shows that the majority of hospitalized COVID-19 survivors, particularly women, still experience symptoms six months after initial infection, and have a decreased quality of life. These findings call for active rehabilitation of COVID-19 patients and highlight the difference in recovery between men and women.
Acknowledgements
We thank Paula Taimi from the Skin and Allergy Hospital for her assistance in patient recruitment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Finnish Institute for Health and Welfare. Situation update on coronavirus [cited 2021 Mar 1]. Available from: https://thl.fi/en/web/infectious-diseases-and-vaccinations/what-s-new/coronavirus-covid-19-latest-updates/situation-update-on-coronavirus
- Helsinki University Hospital. Coronavirus in numbers [cited 2021 Mar 1]. Available from: https://www.hus.fi/en/newsroom/coronavirus-covid-19/coronavirus-numbers
- World Health Organization. In the wake of the pandemic: preparing for long COVID. Policy Brief 39. 2021. Available from: https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/publications-and-technical-guidance/2021/in-the-wake-of-the-pandemic-preparing-for-long-covid-2021
- Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27(1):89–95.
- Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232.
- Hui DS, Wong KT, Ko FW, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128(4):2247–2261.
- Ngai JC, Ko FW, Ng SS, et al. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15(3):543–550.
- Ong KC, Ng AW, Lee LS, et al. 1-Year pulmonary function and health status in survivors of severe acute respiratory syndrome. Chest. 2005;128(3):1393–1400.
- Daher A, Balfanz P, Cornelissen C, et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir Med. 2020;174:106197.
- Spruit MA, Holland AE, Singh SJ, et al. COVID-19: interim guidance on rehabilitation in the hospital and post-hospital phase from a European respiratory society and American thoracic society-coordinated international task force. Eur Respir J. 2020;56:2002197.
- George PM, Barratt SL, Condliffe R, et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75(11):1009–1016.
- Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2(3):217–227.
- RAND Corporation. 36-Item Short Form Survey (SF-36) Scoring Instructions [cited 2020 Dec 7]. Available from: https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/scoring.html
- Frija-Masson J, Debray M-P, Gilbert M, et al. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur Respir J. 2020;56:2001754.
- Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID-19 – an observational prospective multi-center trial. Eur Respir J. 2021;57(4):2003481.
- Zhu J, Zhong Z, Ji P, et al. Clinicopathological characteristics of 8697 patients with COVID-19 in China: a meta-analysis. Fam Med Community Health. 2000;8:e000406.
- Rozenberg S, Vandromme J, Martin C. Are we equal in adversity? Does COVID-19 affect women and men differently? Maturitas. 2020;138:62–68.
- Chan KS, Zheng JP, Mok YW, et al. SARS: prognosis, outcome and sequelae. Respirology. 2003;8 Suppl:S36–S40.
- Hawryluck L, Gold WL, Robinson S, et al. SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis. 2004;10(7):1206–1212.
- Viegi G, Paoletti P, Prediletto R, et al. Carbon monoxide diffusing capacity, other indices of lung function, and respiratory symptoms in a general population sample. Am Rev Respir Dis. 1990;141(4 Pt 1):1033–1039.