To the Editor,
The prompt recognition of COVID-19 infected patients is essential to control the COVID-19 pandemic. The reverse transcriptase-polymerase chain reaction (RT-PCR) test is the gold standard for diagnosing COVID-19. However, RT-PCR is time-consuming, high cost, and requires trained personnel, making it less approachable. The rapid antigen test is a reasonable alternative with an affordable price and short turnaround time [Citation1]. Recently in this journal, an evaluation of a rapid SARS-CoV2 antigen test, CerTest SARS-CoV2 Card Test, was reported [Citation2]. Among 240 nasopharyngeal swab samples, 80 were RT-PCR positive. In comparison to RT-PCR, the antigen test showed a specificity of 100% and a sensitivity of 79%. Among 63 samples with concordant results, the average RT-PCR Ct was <20, as compared to a median Ct of 23.4 among 17 discordant samples.
We here report our experience with PanbioTM, an alternative rapid antigen test. Data were collected retrospectively from patients who underwent COVID-19 examinations at MacKay Memorial Hospital from May 2021 to November 2021. MacKay Memorial Hospital is a COVID-19-designated hospital. Since the start of the COVID-19 pandemic, it developed well-organized screening methods and established quarantine wards, as previously reported [Citation3,Citation4]. Patients aged ≥20 were included in this study. Two nasopharyngeal samples were retrieved from all patients, one from each nostril. One sample was analyzed by the PanbioTM COVID-19 rapid antigen test, and the other underwent RT-PCR examination (Cobas® SARS-CoV-2). The primary outcome of this study was the sensitivity, specificity, positive predictive value (PPV), and a negative predictive value (NPV) of PanbioTM COVID-19 rapid antigen test compared with RT-PCR in the included study population. The secondary outcome was the performance evaluation of rapid antigen test in high viral load patients compared with RT-PCR. We defined a high viral load as the cycle threshold (Ct) ≤25 upon RT-PCR examinations [Citation5]. The design and execution of this study were approved by the Institutional Review Board of MacKay Memorial Hospital (22MMHIS063e).
A total of 9174 patients were included in this study. Their average age was 44.5 years old, and 49.5% of the patients were male. Of these, 65 patients tested positive by RT-PCR (prevalence: 0.71%), 15 (23%) of PanbioTM COVID-19 rapid antigen test revealed positive results, and 12 cases were rapid antigen test positive/RT-PCR negative. The overall sensitivity and specificity were 23.1% (95% confidence interval [CI]: 13.53%, 35.19%) and 99.9% (95% CI: 99.77%, 99.93%), respectively. This concluded a PPV of 55.3% (95% CI: 37.57%, 71.70%) and NPV of 99.5% (95% CI: 99.38%, 99.53%). Moreover, we also accessed the performance of rapid antigen test in patients with high viral loads. In 19 patients, their COVID-19 RT-PCR revealed Ct ≤25. Of these, 15 tests were rapid antigen test positive, and four were rapid antigen test negative. The sensitivity and specificity in high viral load patients were 79.0% (95% CI: 54.43%, 93.95%) and 99.9% (95% CI: 99.77%, 99.93%), respectively. The PPV and NPV were 54.7% (95% CI: 39.58%, 68.98%) and 99.9% (95% CI: 99.90%, 99.98%), respectively. We also discovered a significantly lower Ct value (p<.001) in the rapid antigen test (+) group than in the rapid antigen test (-) group ().
Figure 1. RT-PCR cycle thresholds of COVID-19 patients testing either positive or negative by PanbioTM COVID-19 rapid antigen test.
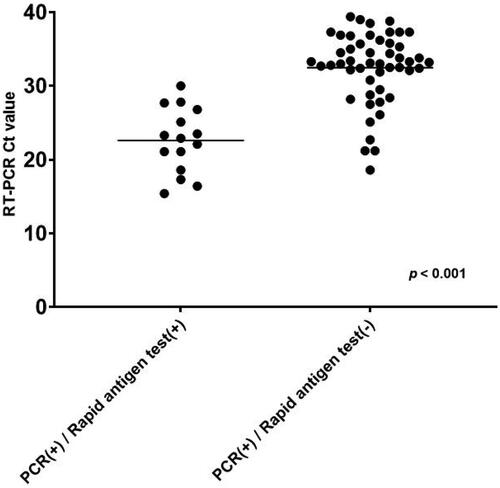
As far as we know, this study contained the largest study population of PanbioTM COVID-19 rapid antigen test in a real-world situation. A low overall sensitivity was observed in this study, which was similar to previous studies conducted in a low prevalence population [Citation6,Citation7]. Owing to the extremely low prevalence in this study, NPV was 99.5%. However, a remarkable increase in sensitivity was noticed for samples with Ct ≤25, which was regarded as a transmitted threshold for COVID-19 [Citation5]. We noticed that the results in this study might vary since Taiwan was once viewed as one of the few successful countries to contain the notorious virus. However, this study indicated that the test results of PanbioTM COVID-19 rapid antigen test should be interpreted carefully in different epidemiological settings. In a low prevalence community, negative results should be believed, whereas a positive rapid antigen test result might require further RT-PCR examination for confirmation. On the contrary, a positive PanbioTM COVID-19 rapid antigen test result should be admitted in a high prevalence community, whereas patients with negative results might receive RT-PCR when COVID-19 infection remains highly suspected.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Navero-Castillejos J, Casals-Pascual C, Narvaez S, et al. Diagnostic performance of six rapid antigen tests for SARS-CoV-2. Microbiol Spectr. 2022;10(2):e0235121.
- Trobajo-Sanmartin C, Navascues A, Miqueleiz A, et al. Evaluation of the rapid antigen test CerTest SARS-CoV-2 as an alternative COVID-19 diagnosis technique. Infect Dis. 2021;53(9):730–732.
- Chen T-H, Chi W-Y, Yang S-Y, et al. The role of quarantine ward in the COVID-era: a Taiwan medical center experience. Health Technol. 2021;5:9–9.
- Tsai Y-L, Chen T-H, Chi W-Y, et al. Screening unit during coronavirus disease 2019 pandemic: a review of current evidence and experience of a medical center in Taiwan. Health Technol. 2021;5:7–7.
- Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469.
- Fenollar F, Bouam A, Ballouche M, et al. Evaluation of the panbio COVID-19 rapid antigen detection test device for the screening of patients with COVID-19. J Clin Microbiol. 2021;59(2):e02589–20.
- Torres I, Poujois S, Colomina AE, et al. D. Evaluation of a rapid antigen test (panbio COVID-19 Ag rapid test device) for SARS-CoV-2 detection in asymptomatic close contacts of COVID-19 patients. Clin Microbiol Infect. 2021;27(4):636 e1–636 e4.
