Abstract
Membrane topography of living cells has been considered as an effective parameter that reflects cellular statuses. With the improvements in spatial and temporal resolutions of various measurement techniques, the changes of membrane topography in response to various external stimulations in the culture environments can be accurately recorded. Membrane roughness is a useful parameter to evaluate the changes in membrane topography. At present, atomic force microscopy (AFM) is the most common technique to measure the membrane topography and roughness of living cells. On the other hand, with the non-contact profiling capability, many optical techniques are also employed in this field of research. This review briefly introduces the evolution and applications of AFM on measuring membrane roughness. Meanwhile, quantifying the cell membrane topography and roughness with a non-scanning, non-contact, and label-free optical technique, non-interferometric wide-field optical profilometry (NIWOP), is also presented.
1. Introduction
Living cells are bordered by consecutive lipid bilayers, as known as the cell membranes. Most of cellular receptors and sensors are located on the membranes to play indispensable roles in various physiological functions. Hence, external stimulations on cells and consequent cellular responses could vary the membrane curvature and mechanics significantly [Citation1]. For example, the membrane roughness has been proposed as a parameter that can reflect the cellular status under various treatments measured by atomic force microscopy (AFM) [Citation2–5]. However, the AFM measurement on a single cell usually requires several tens of minutes to get a sufficiently large field-of-view image with enough sampling positions. High-speed AFM (HS-AFM) was employed to measure the dynamic conformation variations of biological macromolecules at much faster imaging rates [Citation6,7]. Even the temporal resolution is largely improved, the collisions between the AFM cantilever tip and the cell membranes are inevitable, which results in possible damages to the samples. HS-AFM equipped with a several-micrometer-long tip was invented to overcome this drawback and could be more applicable on living cells [Citation8].
In addition to the conventional AFM measurements on cell surface topography, in this review we introduce a label-free and non-contact optical method, non-interferometric wide-field optical profilometry (NIWOP), and its applications on measuring membrane topography and characterizing membrane roughness. This optical technique can provide depth resolution up to 20 nm [Citation9], which is sufficient for quantifying the variations of cell membrane topography relevant with the change of cytoskeleton configurations. AFM studies on cell membrane roughness revealed that the variations in the tens to hundreds of nanometers scale are important features for cellular responses to external treatments [Citation2,4,5,10]. Compared with conventional AFM, NIWOP possesses the advantages of wild-field profiling and fast open-loop recording, which are useful for unraveling dynamic responses of cell membranes and accumulating sufficient data for statistical analyses in a reasonable period. For example, the propagation of membrane ripples near the cell edges was observed by NIWOP with an imaging speed as high as 5 frames/min while the underlying cytoskeleton structure was investigated [Citation9,11,12]. The time-lapsed internalization process of gold nanoparticles in living cells [Citation13,14] and cell membrane deformations under the modulation of piconewton external force [Citation15] were also demonstrated by the NIWOP observation.
In this review, we also introduce the recent and relevant literatures dealing with membrane roughness as indicators for cells in response to external triggers, including chemical, physical, and drug treatments.
2. Surface topography of living cells
At present, several kinds of instruments can profile sample surfaces with nanometer spatial resolution. However, some of them such as scanning electron microscopes are not suitable for manipulating living cells in physiological conditions. Moreover, because the membrane topography of living cells is dynamic, only a few techniques could fit the requirements for the investigations on membrane topography variations under external stimulations. Here, we discuss AFM and NIWOP as representative nanoprofiling techniques.
2.1. AFM studies on cell membranes
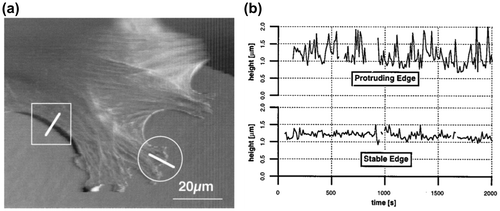
AFM is an excellent tool for imaging surface topography of objects because it reaches the resolution of atoms on solid surfaces [Citation16]. AFM imaging could also be performed in aqueous solutions. So far, AFM has been routinely used for morphological studies on biological samples, such as proteins [Citation17], nucleic acids [Citation18], lipid bilayers [Citation19], and live cells [Citation20]. In addition to the surface topography, AFM could provide the mechanical properties of the sample, such as the elasticity of cells [Citation21]. However, the scanning speed of conventional AFM limits its application from unraveling dynamic biological processes. HS-AFM was developed with much higher imaging speeds that are capable of visualizing the motions of biological molecules in great details [Citation22]. For example, the stepwise motion of myosin V on an actin filament was recorded with the temporal resolution at 3 frames/s [Citation23]. However, because the thickness variations of a common animal cell are in the range of tens of micrometers, collisions between cell body and the AFM tip is inevitable during the image acquisition. When high-speed scanning is conducted, the collisions on the cell body could cause severe damages on the cell membranes. For obtaining more reliable data on living cells, to reduce the scan areas or to use extra-long tips [Citation8] has been proposed as effective solutions for AFM. Figure demonstrates a typical image of cell membrane measured by AFM and the height variations with time at the protruding edge and stable edge, respectively [Citation24]. Nonetheless, conducting AFM measurement on living cells requires expertise and experiences. Ukraintsev et al. summarized various artifacts in AFM measurement on biological samples, including those caused by the tip, scanner, and the interaction force between the tip and sample surface in Ref. [Citation25].
Figure 1. (a) Topographic image of a living fibroblast measured by AFM. (b) The height variations with time at the protruding edge and the stable edge which were marked individually as circle and rectangular area in (a).
2.2. Optical profilometry studies on cell membranes
Conventional far-field optical microscopy has limited lateral resolution owing to the diffraction of light. However, in the field of surface profilometry, the depth sensitivity of optical waves can reach the nanometer scale easily. Therefore, a number of optical profiling techniques have been used to quantify the thickness or membrane topography of biological cells. A direct implementation is to apply interferometric techniques to achieve nanometer depth resolution in profiling cell surface. For example, digital holographic microscopy can be used to measure cell thickness and volume, provided that the refractive indices of the cytoplasm can be determined with additional optical information [Citation26,27]. A technique called live-cell interferometry has been used to study the variations of cellular thickness under the stimulation of external force [Citation28]. Phase-shifted laser feedback interference microscopy has also been used to measure the topography of fixed and living cells [Citation29]. However, the variations of the intracellular indices of refraction at different locations in a living cell could bring uncertainties for optical phase-based membrane height measurements. One could fix and coat the cell with high-reflectivity metals, and then use optical interferometer to obtain the true membrane topography [Citation30,31]. But in this way, dynamical membrane activities cannot be recorded. Meanwhile, acoustic microscopy [Citation32] and photoacoustic microscopy [Citation33] have also been employed to obtain the cellular thickness of living cells. However, at present the depth resolution of these acoustical techniques remains in the micrometer scale.
Without the phase measurement in optical interferometry, other optical techniques can still obtain nanometer depth sensitivity. An example is differential confocal microscopy (DCM) [Citation34,35], which achieves nanometer depth sensitivity by placing the sample surface away from the focal plane, into the sharp slope region of the axial response curve of confocal microscopy. DCM provides depth resolution better than 10 nm on solid-state materials using standard optical components. Furthermore, because DCM is a far-field optical technique, it is also useful for measuring the perturbations on soft biological membranes in aqueous solutions [Citation36,37]. The principle of DCM has been implemented with various setups. For example, a fiber-based DCM system was proposed to reduce the footprint and to improve the stability in comparison with the conventional pinhole-based configuration [Citation38]. DCM could also be realized in a digital approach by processing a series of through-focus images obtained by a confocal microscope [Citation39]. A two-detector DCM scheme for real-time elimination of the reflectivity effects from the sample surface was proposed [Citation40]. Recently, a setup using a chromatic objective and a broadband light source to produce a 30-μm measurement dynamic range and to perform one-shot line profiling in DCM was demonstrated [Citation41].
Structured illumination is another skill to obtain optically sectioned images similar to those acquired by confocal microscopy [Citation42]. In comparison with confocal microscopy, one of the advantages of structured illumination optical sectioning microscopy is that the data could be obtained without lateral scanning mechanisms. This feature is especially appreciated in the studies on membrane activities in living cells. NIWOP [Citation43] is a technique combining the concepts of DCM and structured illumination optical sectioning microscopy. On solid-state surface with adequate reflectivity, NIWOP provides sub-10-nm height profiling sensitivity with conventional microscope objectives. On cell membranes, a simple data processing was developed to remove the signal contributed from the reflection on the cell bottom interface, and membrane topography on the apical side of a cell could be obtained [Citation9].
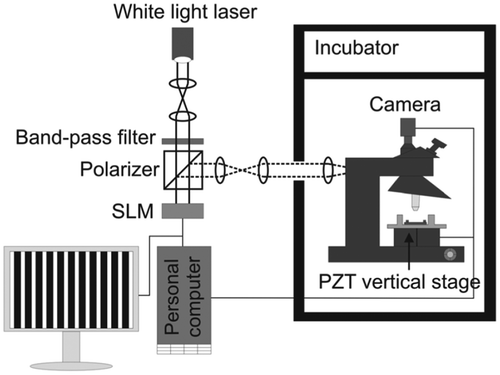
Figure shows a typical setup of the NIWOP system which is useful for long-term recording of the cell membrane topography. In brief, three image frames (I1, I2, I3) of the same sample with the projected grid pattern shifted by one-third of the pattern period are captured. Then the square-law detection principle: was used to remove the grid pattern and obtained the sectioned image Is. The membrane height at each pixel on the sample surface is obtained with a calibration processing [Citation9]. In the NIWOP system used in Ref. [Citation44] and Ref. [Citation45], the three image frames were recorded within five seconds, such that the temporal resolution was about 12 images/min.
Figure 2. Schematic diagram of the setup of an NIWOP system that is used for long-term recording of cell membrane topography.
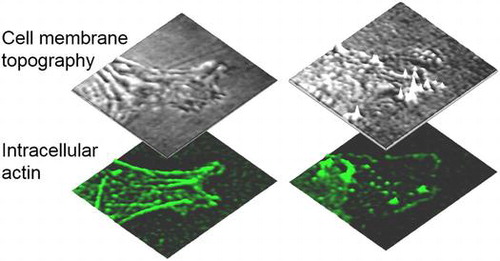
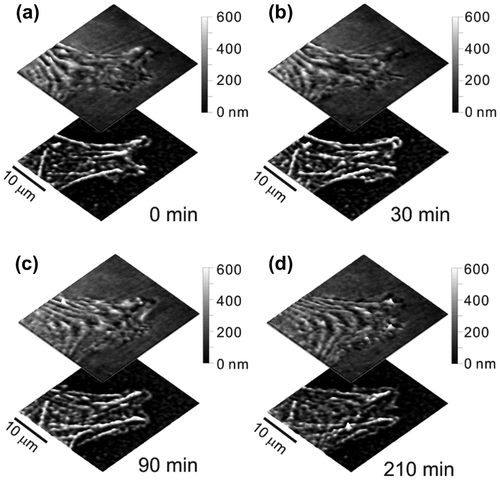
Because the NIWOP setup is constructed on a conventional optical microscope, the same system can also be used for correlative fluorescence imaging. Figure demonstrates that the NIWOP system could be used to monitor the dynamics of the membrane as well as the underlying actin cytoskeletons on the same cell. In this way the topographical features could be correlated with the configurations of the actin cytoskeletons. This function is especially useful for the studies related to cell migration and cytoskeleton organization. For example, Chen et al. measured the propagation characteristics (amplitudes, wavelengths, dispersion relation, etc.) of membrane ripples on living human dermal fibroblast, and suggested that these membrane ripples are supported by the contractile force of myosin II and the protrusion force from actin polymerization [Citation12]. The measurement results compared well with the active membrane wave model proposed by Shlomovitz and Gov [Citation46,47].
Figure 3. Time-lapse images of the membrane topography and actin in a lamellipodium of a living fibroblast.
3. Applications of membrane topography measurements
3.1. Monitoring the membrane responses to external stimulations
Membrane activities are closely related to many cellular functions, including cell migration, cytoskeleton protrusion, endocytosis, receptor clustering [Citation48], and therefore should also be sensitive to the stimulations from the culture environment. Recently, with the advances of surface-profiling techniques, scientists got evidences to show that membrane roughness is closely related to external treatments. Conventionally, membrane roughness is calculated in two ways: (1) Average roughness, defined as , where hi is the measured membrane height at position i,
is the average membrane height, and n is the number of measured values. (2) Root-mean-squared roughness, defined as
[Citation4,49]. In optical image-based measurement, because the pixel numbers could be several hundreds to thousands in a field of view, one may also use the standard deviation of the heights measured at all pixels as the membrane roughness [Citation45]. In every case, membrane topography must be obtained before the calculation of membrane roughness. Therefore, membrane roughness can be regarded as one kind of quantification representations of membrane topography.
On murine fibroblast 3T6, Risuleo et al. found that membrane roughness decreased after the interaction with cationic liposomes (DMPC–ge1 mixture, diameters around 110–120 nm), measured by AFM [Citation50]. Figure shows the tapping-mode AFM images of cells before and after the treatment with the liposomes. The authors suggested that the smoothening of cell surface should result from the significant swelling of the cells with the internalization of the cationic liposomes. This work demonstrated that the membrane roughness could be changed by particles with specific electrical charges in the culture environment. Lee et al. conducted a similar observation on the interaction between the mouse neuroblastoma cell N2a and gold nanoparticles (diameter ~30 nm) with NIWOP. The treatment of positively charged gold nanoparticles reduced the membrane roughness by nearly 14%, while the negatively charged gold nanoparticles did not show a significant effect. This result could be related to the higher adsorption of the positively charged nanoparticles onto the cell membrane, which was also confirmed with images obtained with scanning electron microscopy [Citation45].
Figure 4. Tapping-mode AFM images and corresponding surface profiles of 3T6 fibroblasts. (A,B) An untreated cell. (C,D) A cell incubated with cationic liposomes.
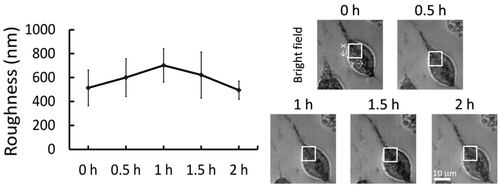
Using the NIWOP technique, Lee et al. also showed the temporal variation of the N2a cell membrane roughness after the treatment with a hypertonic solution (75 mM sucrose in the culture medium). In Figure we can see that the membrane roughness increased within the first hour of the treatment, but it gradually dropped to the initial status when the cell became adapted to the environmental variation [Citation45].
Figure 5. The left diagram shows the temporal variation of membrane roughness induced by a hypertonic solution (75 mM sucrose in the culture medium).
3.2. Discriminating ill cells from healthy ones
Membrane topography of living cells depends heavily on the mechanical properties of membranes as well as the structures and organizations of cytoskeletons. Several diseases influence the functions of various cytoskeleton proteins [Citation51–53], such that they often change cell shapes and the ultra-structures of cell membranes. Buys et al. showed that the membrane roughness of red blood cells (RBCs) obtained from diabetes patients were correlated with the disease status [Citation54]. The results were measured from fixed cells by AFM and confirmed with scanning electron microscopy. The RBCs from diabetic patients showed significant differences in shape, size, and membrane roughness, in comparison with those from healthy people. The results are summarized in Table .
Table 1. Statistical values of normal and diabetic red blood cell morphologies measured by AFM.
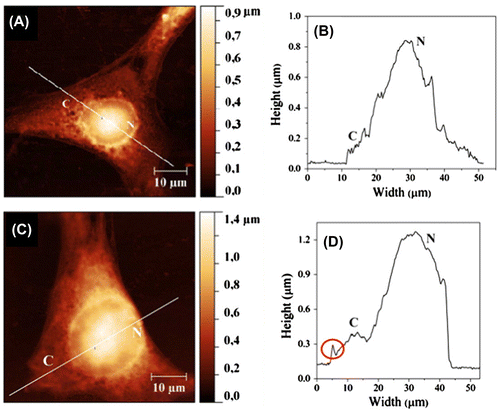
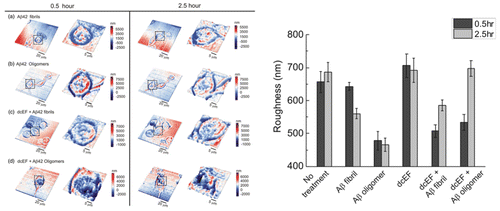
Membrane properties are also relevant with neurodegenerative diseases. For example, amyloid-beta (Aβ) peptides are found to exist in brain lesions of Alzheimer’s disease (AD) patients and are considered as an indicator for the progression of AD. Aβ peptides are easily assembled with various degrees of polymerization and form different conformations. Both Aβ fibrils and oligomers are demonstrated to be neurotoxic, and Aβ42 is believed to be related to the development of AD by perturbing the properties and functions of cell membranes [Citation55,56]. Using AFM, Lulevich et al. found that the oligomers of Aβ42 increased the stiffness of N2a cell more significantly than the fibrils [Citation57]. Based on the NIWOP measurement, Pan et al. demonstrated that both Aβ42 fibrils and oligomers reduced the membrane roughness of N2a cells, and the effect of Aβ42 oligomers was stronger and faster than that of the fibrils [Citation44]. It seemed that higher cell stiffness correlated with smaller membrane roughness. Interestingly, with the application of a 300 mV/mm direct current electric field (dcEF), the membrane roughness could be recovered under the treatment of Aβ42. But the mechanisms are unknown. The NIWOP images and the statistics of membrane roughness variations are shown in Figure .
Figure 6. Topographic images (left) and the statistics of membrane roughness variations (right) of N2a cells measured by NIWOP at 0.5 and 2.5 h under various external treatments.
The N-methyl-D-aspartate (NMDA) receptor is one of the main glutamate receptors in mammalian central nervous systems. The excessive activation of NMDA receptors facilitates Ca2+ influx into neurons, and then causes the diseases such as Huntington’s disease [Citation58] and AD [Citation59]. Fang et al. employed AFM to investigate the human neuroblastoma cell SH-SY5Y under the treatments of NMDA. A significant surface roughness increase (~28%) was measured and the time-dependent stiffness variations were observed [Citation4]. They also used MK801 (dizocilpine), an inhibitor of the NMDA receptors, to block the NMDA receptors and reduced the Young’s modulus back to that of the control group. This work also provided the evidences that the NMDA receptor-medicated neurodegenerative process is related to the mechanical properties of neuronal cells.
Even though the detailed cellular mechanisms of the neurodegenerative diseases remain to be investigated, the previous works indicated that membrane roughness is correlated with the degrees of illnesses. Therefore, membrane roughness could be a useful parameter for figuring out detailed mechanisms or evaluating the effects of various types of therapeutic treatments for diseases.
3.3. Cell membrane responses to chemical stimulations
As discussed above, membrane roughness of living cells is a measurable and sensitive parameter for detecting the cellular responses to external stimulations. Therefore, we might use the measurement on membrane roughness as an assay to evaluate the effects of certain chemicals on the targeted cells. Wang et al. used AFM to detect the changes of membrane roughness on RAW 264.7 cells (mouse monocyte/macrophage) at the early stage of apoptosis induced by H2O2 [Citation2]. They found that the membrane roughness increased with the H2O2 treatment in a dose-dependent manner. Kim et al. discovered that Ishikawa (human endometrial adenocarcinomas) and HeLa cells exhibited higher membrane roughness and lower cell stiffness with the treatment of paclitaxel (Taxol) [Citation10]. Recently, Kung et al. also found that the membrane roughness of B16-F10 melanoma cells increased in response to cisplatin-mediated cell death [Citation5]. All these AFM measurements were conducted on fixed cells. In general, the fixation procedures, including chemical or physical methods, would change cell morphologies and mechanical properties. Aldehyde-based fixatives are useful for conserving cell or bacterial morphologies [Citation60,61]. Nonetheless, it is very possible that different samples require different optimization mixtures of fixatives. Although the measurements on fixed cells might not provide completely native cell membrane morphologies, they can still show the trends of topographical variations under specific treatments.
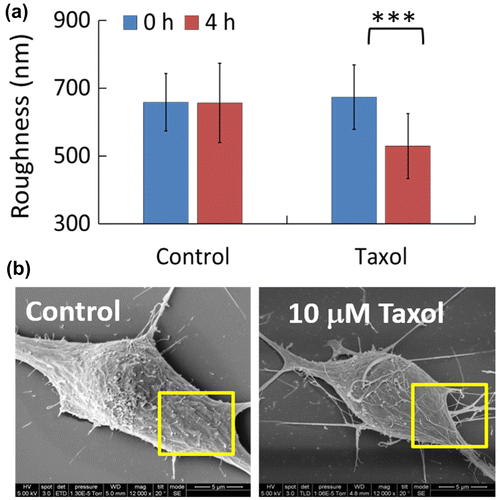
Direct measurements on living cells are the most desirable ways if we would like to use membrane topography as a diagnostic parameter for cellular statuses under external stimulations. Using the NIWOP technique, Lee et al. tested the effect of 10 μM paclitaxel on the membrane roughness of living N2a cells [Citation45]. Although the drug did not cause any significant morphology changes and cell death, it decreased the membrane roughness significantly. This topography change on the cell membrane was confirmed by scanning electron microscopy imaging, as shown in Figure . The smoother membranes of the N2a cell might result from the stabilization [Citation62,63] and redistribution of the microtubules induced by paclitaxel.
Figure 7. (a) Membrane roughness of N2a cells under the treatment of 10 μM paclitaxel (Taxol) for 4 h, measured by NIWOP. (b) Scanning electron microscopy images of the N2a cells.
The studies with both AFM and NIWOP have demonstrated that membrane roughness is an effective indicator when cells are treated with chemicals or drugs. Membrane roughness could thus be considered as a useful parameter to evaluate the efficacy of potential drugs or drug combinations at the early stages of testing. Because the optical measurement of membrane roughness is label-free and the illumination optical intensity is low, the cell viability would be preserved better than the methods based on fluorescence microscopy.
4. Conclusions
With the improvements of nanoprofiling techniques, membrane topography of living cells is now measurable with sufficiently high spatial and temporal resolutions. Therefore, parts of the underlying mechanisms determining the membrane topography and roughness are becoming clearer. Mechanobiology is thought to be closely related to the developments of many diseases, including cancers and neurodegenerative diseases. Cross et al. have indicated that metastatic cancer cells are softer than normal cells by more than 70% [Citation64]. It is also illustrated that both internal and external physical forces can act through the cytoskeletons to alter the cellular behaviors [Citation65]. The cell membranes closely connected to the underlying cytoskeletons must be altered as well. Therefore, the membrane topography could be used for cellular diagnostics in different stages of disease developments.
Because cells also receive the stimulations of multiple factors from neighboring ones, we also expect that the measurement on cell membrane topography would be conducted in co-culture systems. In this way, the mechanical properties or roughness of cell membranes could also be used as one feature related to cell–cell interactions. The membrane mechanoprofiling should find many applications in the studies of current cell biology.
Funding
This work was supported by the Ministry of Science and Technology, Taiwan [grant number 103-2112-M-001-019-MY3]; and the Academia Sinica Research Program on Nanoscience and Nanotechnology.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- H.T. McMahon and J.L. Gallop, Nature 438 (2005) p.590.10.1038/nature04396
- D.-C. Wang, K.-Y. Chen, C.-H. Tsai, G.-Y. Chen and C.-H. Chen, J. Biomech. 44 (2011) p.2790.10.1016/j.jbiomech.2011.08.021
- C. Ke, H. Jin and J. Cai, Scanning 35 (2013) p.316.10.1002/sca.v35.5
- Y. Fang, C.Y. Iu, C.N. Lui, Y. Zou, C.K. Fung, H.W. Li, N. Xi, K.K. Yung and K.W. Lai, Sci. Rep. 4 (2014) p.7074.
- M.-L. Kung, C.-W. Hsieh, M.-H. Tai, C.-H. Weng, D.-C. Wu, W.-J. Wu, B.-W. Yeh, S.-L. Hsieh, C.-H. Kuo, H.-S. Hung and S. Hsieh, Phys. Chem. Chem. Phys. 18 (2016) p.7124.10.1039/C5CP07971C
- T. Ando, N. Kodera, E. Takai, D. Maruyama, K. Saito and A. Toda, Proc. Natl. Acad. Sci. U.S.A. 98 (2001) p.12468.10.1073/pnas.211400898
- M. Shibata, T. Uchihashi, H. Yamashita, H. Kandori and T. Ando, Angewan. Chemie Int. Ed. 50 (2011) p.4410.10.1002/anie.201007544
- M. Shibata, T. Uchihashi, T. Ando and R. Yasuda, Sci. Rep. 5 (2015) p.930.10.1038/srep08724
- C.-C. Wang, J.-Y. Lin and C.-H. Lee, Opt. Express 13 (2005) p.10665.10.1364/OPEX.13.010665
- K.S. Kim, C.H. Cho, E.K. Park, M.-H. Jung, K.-S. Yoon and H.-K. Park, PLoS One 7 (2012) p.e30066.10.1371/journal.pone.0030066
- C.-C. Wang, J.-Y. Lin, H.-C. Chen and C.-H. Lee, Opt. Lett. 31 (2006) p.2873.10.1364/OL.31.002873
- C.-H. Chen, F.-C. Tsai, C.-C. Wang and C.-H. Lee, Phys. Rev. Lett. 103 (2009) p.238101.10.1103/PhysRevLett.103.238101
- C.-C. Wang, C.-W. Lee, C.-Y. Huang, J.-Y. Lin, P.-K. Wei and C.-H. Lee, Appl. Opt. 47 (2008) p.2458.10.1364/AO.47.002458
- C.-C. Wang, C.-P. Liang and C.-H. Lee, Appl. Phys. Lett. 95 (2009) p.203702.10.1063/1.3265920
- C.-C. Wang, H.-J. Jian, C.-W. Wu and C.-H. Lee, Microsc. Res. Tech. 71 (2008) p.594.10.1002/jemt.v71:8
- G. Binnig, C.F. Quate and C. Gerber, Phys. Rev. Lett. 56 (1986) p.930.10.1103/PhysRevLett.56.930
- D.J. Müller, H. Janovjak, T. Lehto, L. Kuerschner and K. Anderson, Prog. Biophys. Mol. Biol. 79 (2002) p.1.10.1016/S0079-6107(02)00009-3
- H.G. Hansma, M. Bezanilla, F. Zenhausern, M. Adrian and R.L. Sinsheimer, Nucleic Acids Res. 21 (1993) p.505.10.1093/nar/21.3.505
- M.-P. Mingeot-Leclercq, M. Deleu, R. Brasseur and Y.F. Dufrêne, Nat. Protocols 3 (2008) p.1654.10.1038/nprot.2008.149
- D.J. Müller, J. Helenius, D. Alsteens and Y.F. Dufrêne, Nat. Chem. Biol. 5 (2009) p.383.10.1038/nchembio.181
- A. Touhami, B. Nysten and Y.F. Dufrêne, Langmuir 19 (2003) p.4539.10.1021/la034136x
- T. Ando, T. Uchihashi and N. Kodera, Ann. Rev. Biophys. 42 (2013) p.393.10.1146/annurev-biophys-083012-130324
- N. Kodera, D. Yamamoto, R. Ishikawa and T. Ando, Nature 468 (2010) p.72.10.1038/nature09450
- C. Rotsch, K. Jacobson and M. Radmacher, Proc. Natl. Acad. Sci. U.S.A. 96 (1999) p.921.10.1073/pnas.96.3.921
- E. Ukraintsev, A. Kromka, H. Kozak, Z. Remeš and B. Rezek, Artifacts in atomic force microscopy of biological samples, in Atomic Force Microscopy Investigations into Biology – From Cell to Protein, C. Frewin, ed., InTech, Rijeka, 2012, p.29.
- P. Marquet, B. Rappaz, P.J. Magistretti, E. Cuche, Y. Emery, T. Colomb and C. Depeursinge, Opt. Lett. 30 (2005) p.468.10.1364/OL.30.000468
- B. Rappaz, F. Charrière, C. Depeursinge, P.J. Magistretti and P. Marquet, Opt. Lett. 33 (2008) p.744.10.1364/OL.33.000744
- J. Reed, J.J. Troke, J. Schmit, S. Han, M.A. Teitell and J.K. Gimzewski, ACS Nano 2 (2008) p.841.10.1021/nn700303f
- E. Atilgan and B. Ovryn, Biomed: Opt. Express 2 (2011) p.2417.10.1364/BOE.2.002417
- O.F. Zouani, C. Chanseau, B. Brouillaud, R. Bareille, F. Deliane, M.-P. Foulc, A. Mehdi and M.-C. Durrieu, J. Cell Sci. 125 (2012) p.1217.10.1242/jcs.093229
- O.F. Zouani, V. Gocheva and M.-C. Durrieu, PLoS One 9 (2014) p.e97855.10.1371/journal.pone.0097855
- E.M. Strohm, G.J. Czarnota and M.C. Kolios, I.E.E.E. Trans. Ultrason. Ferroelectr. Freq. Control 57 (2010) p.2293.
- B. Dong, H. Li, Z. Zhang, K. Zhang, S. Chen, C. Sun and H.F. Zhang, Optica 2 (2015) p.169.10.1364/OPTICA.2.000169
- C.-H. Lee and J. Wang, Opt. Commun. 135 (1997) p.233.10.1016/S0030-4018(96)00642-6
- C.-W. Tsai, C.-H. Lee and J. Wang, Opt. Lett. 24 (1999) p.1732.10.1364/OL.24.001732
- C.-H. Lee, C.-L. Guo and J. Wang, Opt. Lett. 23 (1998) p.307.10.1364/OL.23.000307
- C.-H. Lee, W.-C. Lin and J. Wang, Phys. Rev. E 64 (2001) p.020901(R).10.1103/PhysRevE.64.020901
- I. Ilev, R. Waynant, I. Gannot and A. Gandjbakhche, Rev. Sci. Instrum. 78 (2007) p.093703.10.1063/1.2777173
- J. Liu, Y. Wang, C. Liu, T. Wilson, H. Wang and J. Tan, J. Microsc. 256 (2014) p.126.10.1111/jmi.2014.256.issue-2
- L. Qiu, D. Liu, W. Zhao, H. Cui and Z. Sheng, Opt. Express 22 (2014) p.21626.10.1364/OE.22.021626
- L.-C. Chen, D.T. Nguyen and Y.-W. Chang, Opt. Lett. 41 (2016) p.5660.10.1364/OL.41.005660
- M.A.A. Neil, R. Juskaitis and T. Wilson, Opt. Lett. 22 (1997) p.1905.10.1364/OL.22.001905
- C.-H. Lee, H.-Y. Mong and W.-C. Lin, Opt. Lett. 27 (2002) p.1773.10.1364/OL.27.001773
- H.-J. Pan, R.-L. Wang, J.-L. Xiao, Y.-J. Chang, J.-Y. Cheng, Y.-R. Chen and C.-H. Lee, J. Biomed. Opt. 19 (2014) p.011009.
- C.-W. Lee, L.-L. Jang, H.-J. Pan, Y.-R. Chen, C.-C. Chen and C.-H. Lee, J. Nanobiotechnol. 14 (2016) p.119.10.1186/s12951-016-0161-5
- R. Shlomovitz and N.S. Gov, Phys. Rev. Lett. 98 (2007) p.168103.10.1103/PhysRevLett.98.168103
- R. Shlomovitz and N.S. Gov, Phys. Rev. E 78 (2008) p.041911.10.1103/PhysRevE.78.041911
- K. Jaqaman and S. Grinstein, Trends Cell Biol. 22 (2012) p.515.10.1016/j.tcb.2012.07.006
- N.P.B. Au, Y. Fang, N. Xi, K.W.C. Lai and C.H.E. Ma, Nanomed.-Nanotechnol. Biol. Med. 10 (2014) p.1323.
- E. Stefanutti, F. Papacci, S. Sennato, C. Bombelli, I. Viola, A. Bonincontro, F. Bordi, G. Mancini, G. Gigli and G. Risuleo, Biochim. Biophys. Acta-Biomembr. 1838 (2014) p.2646.10.1016/j.bbamem.2014.05.026
- C. McMurray, Cell Death Diff. 7 (2000) p.861.10.1038/sj.cdd.4400764
- F. Ramaekers and F.T. Bosman, J. Pathol. 204 (2004) p.351.10.1002/path.1665
- E. Pretorius and D.B. Kell, Integr. Biol. 6 (2014) p.486.10.1039/c4ib00025k
- A.V. Buys, M.-J. van Rooy, P. Soma, D. Van Papendorp, B. Lipinski and E. Pretorius, Cardiovasc. Diabetol. 12 (2013) p.25.10.1186/1475-2840-12-25
- X. Yang, S. Askarova and J.C. Lee, Mol. Neurobiol. 41 (2010) p.138.10.1007/s12035-010-8121-9
- T.L. Williams and L.C. Serpell, FEBS J. 278 (2011) p.3905.10.1111/j.1742-4658.2011.08228.x
- V. Lulevich, C.C. Zimmer, H.S. Hong, L.W. Jin and G.Y. Liu, Proc. Natl. Acad. Sci. U.S.A. 107 (2010) p.13872.10.1073/pnas.1008341107
- I. Bezprozvanny and M.R. Hayden, Biochem. Biophys. Res. Commun. 322 (2004) p.1310.10.1016/j.bbrc.2004.08.035
- W. Danysz and C.G. Parsons, Int. J. Geriatr. Psychiatr. 18 (2003) p.S23.10.1002/(ISSN)1099-1166
- M. Moloney, L. McDonnell and H. O’Shea, Ultramicroscopy 100 (2004) p.153.10.1016/j.ultramic.2003.12.010
- Y. Chao and T. Zhang, Appl. Microbiol. Biotechnol. 92 (2011) p.381.10.1007/s00253-011-3551-5
- I. Arnal and R.H. Wade, Curr. Biol. 5 (1995) p.900.10.1016/S0960-9822(95)00180-1
- P.A. Theodoropoulos, H. Polioudaki, O. Kostaki, S.P. Derdas, V. Georgoulias, C. Dargemont and S.D. Georgatos, Cancer Res. 59 (1999) p.4625.
- S.E. Cross, Y.-S. Jin, J. Rao and J.K. Gimzewski, Nat. Nanotechnol. 2 (2007) p.780.10.1038/nnano.2007.388
- D.A. Fletcher and R.D. Mullins, Nature 463 (2010) p.485.10.1038/nature08908
