Abstract
The unique linear and non-linear optical (NLO) properties of small noble metal nanoclusters in hybrid systems formed with peptides will be overviewed. Focus is on two aspects based on theoretical concepts. First, enhancement of absorption of biomolecules by small silver clusters in non-scalable regime in which each atom counts will be illustrated on example of peptides involving aminoacids. Second, concept for design of new NLO-phores based on ligated noble metal nanoclusters in which ligands (peptides) have dual role stabilizing metal clusters and inducing non-linearity. Both complementary directions have attractive potential for medical application. The small silver clusters with their unique optical properties and simple linking to peptides should allow optical labelling of functional intracellular proteins opening perspective for development of new sensors. Understanding of leading factors giving rise to large non-linear properties of peptide ligated small silver nanoclusters allows to propose novel ligand-core NLO-phores for bioimaging as well as for other applications.
1. Introduction
Combination of nanotechnology and biotechnology within development of new hybrid materials incorporates characteristic electronic, photonic and catalytic properties of nanoparticles with recognition and selective catalytic properties of biomolecules such as proteins, enzymes and DNA. Optical properties reflect clearly how metallic nanoparticles can influence the response of closely placed molecules [Citation1]. Example of such effect is surface-enhanced Raman scattering with significant enhancement effect [Citation2]. The presence of metallic particles influences strongly linear and non-linear optical (NLO) properties [Citation3,4], in particular fluorescence [Citation5] of hybrid systems.
In the absence of large nanoparticles with plasmonic properties, Dickson et al. have shown that clusters with few silver atoms integrated in biocompatible environment give rise to strong single molecule stokes and antistokes Raman scattering [Citation6]. Emission of the silver nanoclusters within living cells, where silver clusters are bound to nucleolus proteins, has been reported also by Dickson et al. [Citation7]. The small silver clusters are characterised by bright emission occurring on significantly faster time scale than in the case of organic dyes. Therefore, silver nanoclusters are better cell imaging agents. Creation of new silver cluster fluorophores with unique spectral and photophysical properties using DNA microarrays opened the route for new emitters as well as for single molecule spectroscopy and imaging [Citation8,9].
Parallely, we recognised a decade ago the unique optical properties of small noble metal nanoclusters (silver) with molecular-like discrete electronic states, in non-scalable size regime, in which each atom counts [Citation10]. They play a key role for remarkable structure-dependent optical properties of hybrid systems involving biomolecules, e.g. peptides containing aminoacids [Citation11–24]. In the meantime, the nanoclusters have been widely recognised as a good replacement for organic dyes as chromophores in the context of biosensing [Citation16]. The concept is based on relation between nature of excitations of metallic electrons within nanoclusters and π–π* excitation within aromatic rings present in biomolecules as well as in dyes. We have shown that enhancement of absorption as well as its extension in biomolecules is possible to achieve by small noble metal nanoclusters, particularly silver. This is consequence of the interaction between intense intracluster excitations and π–π* excitation within chromophoric parts of biomolecules [Citation22]. The replacement of dyes by nanoclusters has been conceptually clear but realisation of stable hybrid systems, their preparation and application took some time to be developed and achieved.
In this respect, we have followed two directions, silver nanoclusters-biomolecular hybrids can be stabilised by support [Citation25] or silver nanoclusters can be protected and stabilised by ligands [Citation26,27]. In the latter case, the role of peptides is of key importance, since usually ligands, e.g. glutathione (GS), incorporate peptides with metal atoms causing formation of the metallic core. This direction became attractive to us since it represents additional function of peptides as ligands inducing novel optical properties particularly in non-linear regime [Citation28–30]. Therefore, theoretical investigation of NLO properties of ligated silver clusters complements those of hybrid systems in which nanoclusters enhance linear absorption of peptides containing aminoacids. The interplay between the metallic core and protective surrounding gives rise to novel NLO observables, again parallel to substituted acceptor–donor organic dyes but with preferable properties.
Intense activities in the literature have been observed in the case of gold clusters protected by thiolated ligands [Citation31–34] and considerably less of silver nanoclusters [Citation26,27,35,36]. In fact, the experimental preparation of ligated gold nanoclusters is considerably easier due to higher stability than in the case of silver nanoclusters. On the other hand, due to smaller relativistic effects of silver (larger s–d gap) in contrast to gold atoms the localisation of s-electron excitations in silver clusters gives rise to more intense absorption and emission properties than in the case of gold clusters which is preferable for applications. The influence of ligands, especially those containing sulphur has remarkable role in designing hybrids with desired optical properties as will be discussed later.
Noble metal nanomaterials have received large interest in biomedical applications [Citation37,38]. Ultrasmall gold nanoclusters [Citation33,39] protected by peptides or proteins [Citation40] might be useful for addressing some important biological issues [Citation41]. For example, biocompatibility and renal clearance should be considered in designing suitable Au/Ag NCs for biomedical applications [Citation42]. The question concerning toxicity of Ag NP is often raised. Studies have shown that toxicity is both size and shape dependent [Citation43]. However, the protection of silver clusters by biocompatible ligands may prevent toxicity. In fact, cytotoxicity tests on highly fluorescent silver nanoclusters [Citation44] stabilised by glutathione demonstrated viability in the presence of these luminescence probes even at high concentration. Cell studies confirm the uptake of Ag NCs in epithelial lung cancer cells by an endocytotic process. The promising use of ultrasmall gold NCs with glutathione protecting shell has been proposed as a new class of radiosensitisers for cancer radiotherapy which do not damage normal tissues [Citation45]. Moreover, these nanoclusters were found to be efficiently cleared by kidneys. These results show the high potential of fluorescent noble metal nanoclusters for biolabelling and imaging as alternatives to standard fluorescent probes, such as quantum dots and organic dyes.
In addition to promising radiotherapy properties, noble metal NCs display NIR emission [Citation46,47] and allow for imaging within the tissue transparency window. This property along with their multiphoton excitation might permit deep tissue penetration while minimising background fluorescence and scattering problems.
Therefore, the idea of replacing dyes by clusters has been successful, in particularly for using ligands to induce NLO properties [Citation48,49] of noble metal clusters [Citation28–30,50] in analogy to acceptor–donor substitution of organic dyes in context of NLO properties already developed long time ago.
Successful non-linear optics research direction has been initiated by theoretical discovery by M. Göppert-Mayer in which two-photon absorption (TPA) is defined in 1931 [Citation51] as the electronic excitation induced by simultaneous absorption of a pair of photons which has been experimentally demonstrated in 1961 after invention of the laser [Citation52]. Transitions in the range of 700–1300 nm became accessible with femtosecond pulses. Due to a deeper penetration into biological tissue, TPA gained popularity in the biology community in particularly in microscopy. In addition to deeper penetration depths of long wavelengths in tissues, the spatial resolution has been also improved due to multi-photon nature of the light interacting with the medium [Citation53]. Moreover, two-photon excited fluorescence (TPEF) can reduce background fluorescence of the images offering advantages for applications [Citation54]. Therefore, also photo-dynamic therapy with in situ photon conversion has been proposed as alternative to one-photon absorption (OPA) technique.
In this context, engineering of molecular systems and of nanoparticles with the largest TPA and TPEF cross sections has been undertaken. These cross sections can be compared with molecular first hyperpolarizability. In fact, the process of frequency doubling known as second harmonic generation (SHG) relies on the quadratic or first hyperpolarizability in contrast to TPA and TPEF which is connected with the cubic non-linearity or second hyperpolarizability. For example, in the case of SHG asymmetric push–pull chromophores form a standard class of molecular systems while in the case of TPEF process the class of centrosymmetric systems is used [Citation55–57]. Push–pull chromophore systems consist of donor and acceptor end-groups connected by conjugation parts introducing the possibility of large charge transfer upon excitation [Citation58]. Although such dipolar organic dyes present efficient NLO-phores they have several disadvantageous properties such as poor water solubility, high photobleaching and cytotoxicity.
Therefore, design of nanoparticles with large non-linearities such as TPA cross sections became of large interest. In this context, noble metal nanoclusters in which ligand shell protects metal core represent another route for engineering efficient linear optical (LO)-phores [Citation59] as well as NLO-phores [Citation48]. As already mentioned different ligand shell templates such as organic compounds [Citation60,61] or proteins [Citation62], DNA [Citation63] and dendrimers [Citation64] have been proposed. The use of peptides or proteins as templates ensures good biocompatibility and low cytotoxicity making these nanoclusters highly attractive for bioimaging applications [Citation65]. Moreover, these nanoclusters can exhibit giant TPA cross sections in the NIR region. It has been shown that in particularly biocompatible glutathione-capped gold nanoclusters might have large non-linear properties [Citation66,67].
This stimulated us to proceed by designing efficient NLO-phores using silver metal nanoclusters by manipulating relation between ligands and metal core. Here, ligands can be used as replacement of the push-pull concepts used in organic dyes in order to achieve biocompatibility which is necessary for bioimaging applications. In this context, the theoretical design of ligand protected nanoclusters with large two-photon cross sections became very attractive for us. This is based on interplay between ligand and the non-uniform delocalisation of electrons within the metal core giving rise to large transition dipole moments, thus inducing significant non-linear effects. In this context, the question can be raised if the existence of metal core is necessary for achieving large non-linear properties in ligated clusters.
For these reasons, after brief description of theoretical approaches in Section 2, this contribution will focus on two issues. First, the structural and optical properties of the cluster-biomolecule hybrid systems based on understanding of mutual interaction between subunits will be addressed. For this purpose, linear optical properties of silver nanoclusters-peptide hybrids illustrating enhancement role of metallic subunit in context of biosensing will be presented in Section 3. Second, non-linear properties of silver-ligated nanoclusters will be addressed in Section 4, with the aim to provide concept for designing systems with desirable two-photon optical properties needed for successful application in bioimaging. In the case of easy preparable optical materials such as ligated gold clusters the interplay between absence of metallic core and NLO properties will be addressed in Section 5. Finally, Section 6 contains summary and outlook.
2. Theoretical approaches
Density functional theory (DFT) and its time-dependent version (TDDFT) have been used for determination of the structural and optical properties of the silver clusters-peptide hybrid systems in the gas-phase as well as of liganded clusters. The 19-e- relativistic effective core potential (19-e- RECP) from the Stuttgart group [Citation68] taking into account scalar relativistic effects has been employed for the silver atoms in connection with triple zeta plus polarisation (TZVP) AO basis sets [Citation68,69]. Non-local exchange functional with gradient-corrected correlation functional (B3LYP) [Citation70–72] has been employed to determine structures, while its Coulomb-attenuated version (CAM-B3LYP) [Citation73] has been used to obtain optical properties. The semiempirical AM1 method [Citation74] allowed us for an extensive search for structures of the silver hybrid systems based on the simulated annealing method connected with molecular dynamics. The obtained structures were then reoptimised with the described DFT method. In order to find local minima, the vibrational frequencies have been computed.
For determination of NLO properties such as TPA cross sections, the second-order transition moments Sab obtained from the single residue of the quadratic response function (see Ref. [Citation75, Citation76] and reference therein) are used. For this purpose, a sum over state (SOS) expression for TPA transition matrix from the ground state to an excited state
has been employed:
(1)
The frequency of the incident radiation is equal to half the excitation energy from the ground to the excited state, i.e. ω = ωf /2, μa and μb are the Cartesian components of the dipole moment operator μ and ωi and ωf are the frequencies of excitation from to
and
, respectively. The TPA probability for an isotropic system and in the case of linearly polarised light, is defined as:
(2)
The Equation (Equation2(2) ) can be converted to TPA cross section:
(3)
The constancies label fine structure (α), Bohr radius (a0) and the speed of the light (c). The energy of incoming photon is ω [Citation77]. The Lorentzian broadening with width L = 0.1 eV is used.
In order to prevent the TPA cross sections from blowing up near the one-photon resonances one can use the SOSs approach with the damping factor Γ which requires explicit calculation of all transition dipole moments among excited states, as well as between them and the ground state:
(4)
Equation (Equation4(4) ) is orientationally averaged expressions and is valid only for the case where both photons have the same energy Eλ and for linearly polarised photons with parallel polarisation. For this reason, we adapted the SOS approach (Equation (Equation4
(4) )) which we implemented within DALTON programme [Citation78,79] in the framework of the double residue (DR) approach. Therefore, we have been able to correct adequately the TPA cross sections if necessary by damping factor Γ as well as to include necessary manifold of states, usually 20–30 excited states. In this manner we are able to avoid ‘few states’ model in which only the dominating terms in the TPA transition amplitude tensor are included [Citation80]. We tested also the influence of the truncation of the SOS from e.g. 20 to 15 states on the values of cross sections and no influence has been found. Alternative way to correct unphysically large TPA cross sections in the case of resonances between OPA and TPA states is to use damped response theory formalism within TDDFT approach [Citation81].
Computational results should be considered as qualitative, since the influence of different functionals as well as inclusion of double excitations within TDDFT as well as different approaches to correct TPA cross sections close to resonance have not been investigated in details. Nevertheless, the conceptual issues can be addressed using obtained results.
3. Absorption enhancement of peptides by silver clusters
The concept developed for the absorption enhancement of peptides through interaction with silver clusters has been obtained by using model systems changing cluster size as well as length of peptides containing aminoacids such as tryptophan or histidine because of their key biological roles [Citation50]. Moreover, the theoretical concept has been successfully verified by the experimental findings in the gas phase. It is well known that the smallest size silver clusters exhibit unique absorption and fluorescence properties. For example, the silver trimer has been successfully used as an intracellular marker. For this reason we investigated first absorption properties of tryptophan-Ag3+ and dipeptide (TrpGly)-Ag3+ as shown in Figures and . The cationic species have been selected due to their experimental preparation. In both cases, the lowest energy structures corresponds to a charge solvated-like structures where the triangular Ag3+ is coordinated with indole ring of tryptophan. Due to possible contribution of isomers at room temperature the thermal broadening has been taken into account. The spectra for both hybrid systems are similar and are characterised by two bands located around 250 nm and 320 nm. The electronic transitions involving the coupling of the S → Px,y type excitations within Ag3+ with a charge transfer excitation between the Ag3+ and indole ring are responsible for the intense band as shown in Figure . The major charge redistribution after excitations takes place in the Ag3+ according to density difference between the electronic ground and excited state located at 288 nm as shown in Figure . The substationally less intense band arises due to coupling of the third S → Pz excitations within Ag3+ with the charge transfer involving the indole ring and the dipeptide bond.
Figure 1. (a) Calculated TD-DFT photoabsorption spectrum of [Trp·Ag3]+. The lowest energy structure is shown. (b) Thermal broadening of the above absorption spectrum obtained by MD simulations is compared with experimental findings at T≈300 K (red curve). Source: Reproduced from J. Chem. Phys. 125, (2006) p.164326.
![Figure 1. (a) Calculated TD-DFT photoabsorption spectrum of [Trp·Ag3]+. The lowest energy structure is shown. (b) Thermal broadening of the above absorption spectrum obtained by MD simulations is compared with experimental findings at T≈300 K (red curve). Source: Reproduced from J. Chem. Phys. 125, (2006) p.164326.](/cms/asset/510be241-dbcf-4aef-9a7e-4f9f3ce5d5f7/tapx_a_1352458_f0001_oc.gif)
Figure 2. (a) Theoretically simulated thermally broadened absorption spectrum of [TrpGly·Ag3]+ at T = 300 K compared with experimental photofragmentation spectrum (red curve). The black and green sticks correspond to the statistical ensembles of spectra around the most stable isomers I and II, respectively. (b) Thermal ensemble of structures at T = 300 K obtained from constant temperature MD simulations. (c) The analysis of the leading excitations between occupied and virtual Kohn-Sham orbitals participating in the intense transition at 288 nm. HOMO and LUMO label the highest occupied and the lowest unoccupied Kohn-Sham orbitals. (d) Electron density difference between electronically excited state and the ground state at 288 nm corresponding to the dominant optically allowed transition. Source: Reproduced from Phys. Rev. Lett. 101, (2008) p.213001.
![Figure 2. (a) Theoretically simulated thermally broadened absorption spectrum of [TrpGly·Ag3]+ at T = 300 K compared with experimental photofragmentation spectrum (red curve). The black and green sticks correspond to the statistical ensembles of spectra around the most stable isomers I and II, respectively. (b) Thermal ensemble of structures at T = 300 K obtained from constant temperature MD simulations. (c) The analysis of the leading excitations between occupied and virtual Kohn-Sham orbitals participating in the intense transition at 288 nm. HOMO and LUMO label the highest occupied and the lowest unoccupied Kohn-Sham orbitals. (d) Electron density difference between electronically excited state and the ground state at 288 nm corresponding to the dominant optically allowed transition. Source: Reproduced from Phys. Rev. Lett. 101, (2008) p.213001.](/cms/asset/cdcf28c8-d319-4c8c-ae3d-b9924f6dcb8c/tapx_a_1352458_f0002_oc.gif)
The dominant optical absorption originating from the contribution of Ag3+ cluster located around 300 nm is responsible for significant extension and enhancement of the peptide absorption from 250 to 330 nm as shown in Figure . This permits the use of small silver nanoclusters as absorbing optical labels representing a local probe with remarkable advantages over standard organic dyes. The theoretical results are in good agreement with experimental photofragmentation spectra as shown in Figures and . The interpretation of the interaction of the electronic excitations between subunits is confirmed by observed photofragmentations as described in reference [Citation16].
Figure 3. Comparison of calculated absorption spectrum of the pure protonated dipeptide (Trp-GlyH+) and tripeptide (Trp-Ala2H+) (red lines) with absorption spectra of hybrid systems with Ag3+ (a and c) and with Ag9+ (d) clusters (blue line). Broadening of the lines is simulated by the Lorentzian function with a half-width of 20 nm. The calculated oscillator strengths (fe) for hybrid systems are drawn as black sticks. Analysis of transitions labelled by * is given in Figure . (b) Experimental photofragmentation yields for protonated dipeptide (red triangles), bare Ag3+ (black dots) and Trp-GlyAg3+ hybrid (blue dots) as a function of wavelength. The solid lines serve to guide the eye. Source: Reproduced from Phys. Chem. Chem. Phys. 14 (2012) p.9282 with permission from the PCCP Owner Societies.
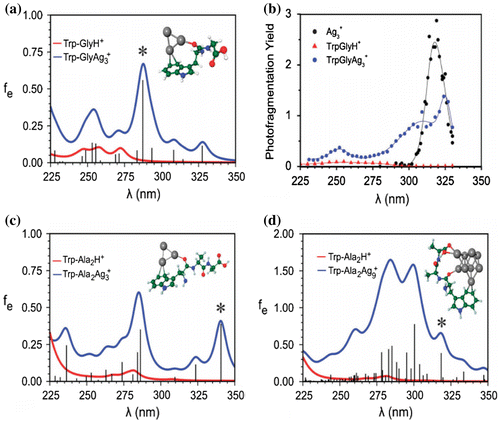
Comparison of absorption spectra of peptides with those obtained for cluster-peptide hybrids shown in Figure allow to rationalise and generalise the enhancement of absorption induced by metallic nanoclusters. The choice of hybrids containing dipeptide Trp-Gly and tripeptide Trp-Ala2 and two cluster sizes Ag3+ and Ag9+ has the purpose to show that enhancement of absorption occurs for both cluster sizes, independent of the peptide length. Moreover, experimental fragmentation yields of the hybrid systems and two isolated subunits TrpGly and Ag3+ shown in Figure (b) allows for comparison with the theoretical results of Figure (a) providing the proof of principle. The lowest energy structures of prototype hybrids systems presented in Figure are of ‘charge solvated nature’ thus, allowing the interaction of excitations within the cluster and within the indole ring, because cationic silver clusters are bound to electron pairs of oxygen atoms and to the π-subunit of the indole ring. In addition, the metal clusters are responsible for substantional reduction of the conformational flexibility of the peptides. Altogether, significant extensions and enhancement of absorption of the peptides from range of 250 nm to near-UV regime present in optical absorption of hybrid systems originate from contribution of silver nanoclusters.
Comparison of experimental fragmentation yields of protonated dipeptide (TrpGlyH+), the free Ag3+ cluster and the spectrum of the hybrid system TryGly-Ag3+ (cf. Figure (b)) confirms the theoretical findings. Absorption band at ~255 nm of protonated Trp-Gly dipeptide is characteristic for Trp-containing peptides due to π-electron excitations within the indole ring. For the silver trimer cation one intense transition at 320 nm has been observed. The experimental photofragmentation spectrum for the hybrid TrpGly-Ag3+ exhibits two fragmentation bands between 225 and 330 nm. The maximum of the second band is lower than in the case of isolated Ag3+ but the photofragmentation is spread over larger range of wavelengths. The agreement between the theoretical and experimental results shown in Figure (a) and (b) is of qualitative nature since the experiments were carried out at the room temperature in contrast to theoretical results obtained at the T = 0 K. The agreement is more quantitative if the temperature broadening is taken into account as shown in Figures and .
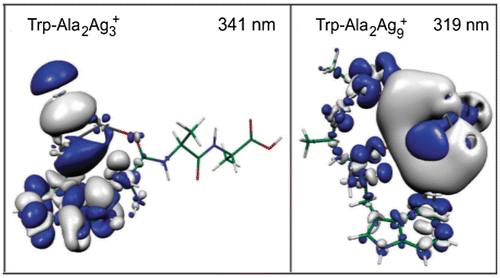
The general concept is illustrated by the absorption spectra calculated for stable structures of hybrid systems for Trp-Ala2-Ag3+ and Trp-Ala2-Ag9+ presented in Figure (c) and (d), allowing to investigate the influence of the peptide lengths and the cluster size on optical properties of hybrids. Comparison of the absorption spectra of both hybrid systems with those of free protonated tripeptide shows strong absorption enhancement and extension in the UV regime due to the presence of the clusters. Moreover, intense transitions in the hybrid system with the Ag9+ cluster are one order of magnitude stronger than in the case of hybrids with Ag3+, illustrating the size effect of a cluster. The analysis of the excitations responsible for the intense transitions based on electron density difference given in Figure shows that major charge redistribution after excitations takes place within the cluster. Therefore, we have drawn the conclusion that the investigation of the silver cluster peptide hybrids strongly suggests that the role of clusters as local probes may be substantially advantageous with respect to organic dyes avoiding the chemical coupling of the dyes with the peptides and most importantly increasing the sensitivity of the detection.
Figure 4. Electron density difference between the electronically excited state and ground state (left) for Trp-Ala2Ag3+ at 341 nm and (right) for Trp-Ala2Ag9+ at 319 nm corresponding to the transitions labelled by * in Figure . Source: Reproduced from Phys. Chem. Chem. Phys., 14 (2012) p.9282 with permission from the PCCP Owner Societies.
4. Ligand – silver core NLO-phores
Over the last decade or more investigation of ligated noble metal clusters where ligand shell has protected metal core served to search for ultrabright metal fluorophores in context of potential for medical application. Since the shell templates contain also metal atoms the core is composed of very small number of metal atoms. Different ligands have been proposed including small organic compounds, proteins, DNA and dendrimers. These protected nanoclusters exhibit intense luminescence with large stokes shift, long lifetimes and good photostability. Peptides or proteins as templates are biocompatible and own low cytotoxicity and therefore such ligated nanoclusters are attractive for bioimaging applications. In particularly, glutathione-capped gold nanoclusters have been widely investigated. In addition, these noble metal nanoclusters show large TPA cross sections in the near-IR (NIR) region [Citation66]. Glutathione-capped gold nanoclusters are biocompatible excellent one- and TPEF contrast agents for live cell imaging as proposed by Polavarapu [Citation67].
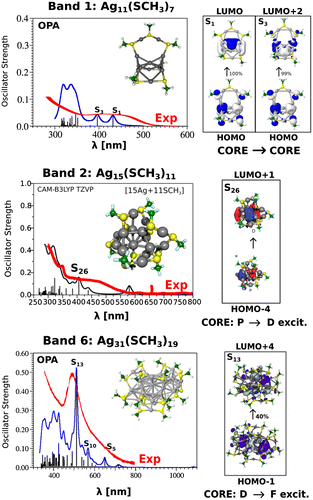
In this context, it is of large importance to develop theoretical concepts allowing to design ligand protected nanoclusters with large TPA cross sections. Quadratic and cubic non-linear processes are based on interplay between the ligands and the non-uniform delocalization of electrons within the metal core depending on its structural properties. For this reason we wish to present linear and non-linear properties of glutathione protected Ag11L7, Ag15L11 and Ag31L19 which have been experimentally prepared and labelled as bands 1, 2 and 6. This stimulated our theoretical investigations which offered the possibility to use peptides as ligands to develop concepts for design of efficient ligand-core NLO-phores.
The smallest cluster Ag11L7 assumes the structure with seven silver atoms forming core containing two tetrahedra with one common central atom in which four electrons are delocalised, two of them in each of two tetrahedra. In the case of Ag15L11 eight silver atoms form the metallic core in which eight electrons are delocalised. For the largest considered cluster Ag31L19, the core is formed by 21 silver atoms and contains 12 delocalised electrons. The theoretical modelling of glutathione is simplified by using SCH3 group. The structures and corresponding TDDFT linear absorption spectra are shown in Figure . The measured and calculated absorption spectra of Ag11L7 and Ag15L11 are similar. They show monotonous increase of intensity with the plateau between 400 and 500 nm. The excited states located within the plateau arise from the excitations within the core as shown in Figure . In contrast, the absorption spectrum for Ag31L19 exhibits large characteristic peak located at 500 nm. This band arises due to core-to-core excitation within the metal core (from D-cluster-core orbital to F-cluster-core orbital) (cf. Figure ). For Ag15L11 and Ag11L7 similar fluorescence spectra have been measured while for Ag31L19 very weak fluorescence has been found.
Figure 5. The DFT structures and TDDFT absorption spectra OPA of Ag11(SCH3)7, Ag15(SCH3)11 and Ag31(SCH3)19 clusters modelling Band 1, Band 2 and Band 6.
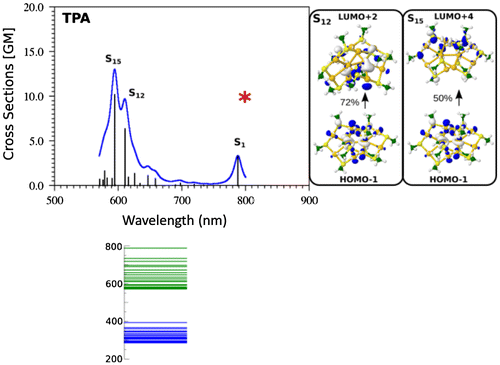
The calculated TPA spectra for all three cluster sizes are shown in Figure and compared with experimental results. Several factors influencing the TPA cross sections have been identified: (i) the excitation between ligands and metal core are characteristic for the non-linear transitions, (ii) the resonance between states involved in the OPA and TPA processes induces large TPA cross sections and (iii) large dipole transition moments are related to a non-uniform electron distribution within the metal core. The role of the structural properties is crucial for determining the electronic distribution within the core. The above listed factors are illustrated in Figures and .
Figure 6. Comparison of TDDFT TPA spectra (left) of Ag11(SCH3)7, Ag15(SCH3)11 and Ag31(SCH3)19 nanoclusters for the lowest energy structures involving 4, 8 and 12 delocalised electrons in the metal core. Source: Reproduced from Nanoscale, 9 (2017) p.1221 with permission from the Royal Society of Chemistry.
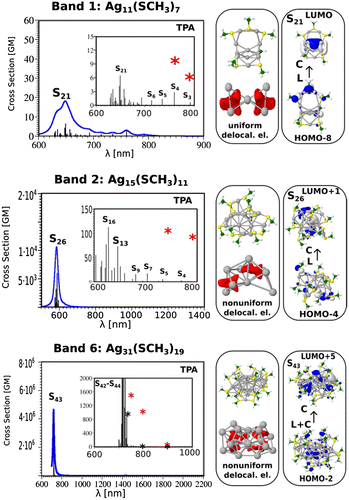
Figure 7. (left part) Comparison of TPA spectra of Ag11(SCH3)7 without and with an external charge on the central Ag atom illustrating an increase of transition dipole moments as well as transition from (right part) uniform delocalised electrons (q = 0) to non-uniform delocalised electrons (q = 0.5). Source: Reproduced from Nanoscale, 9 (2017) p.1221 with permission from the Royal Society of Chemistry.
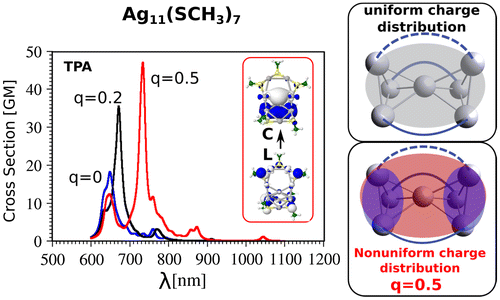
In the case of Ag11L7 the resonance between the OPA process with the excited state S1 localised at wavelengths of 450 nm has not been achieved within the manifold of about 100 states. Moreover, the ELF representing delocalised electrons within the core is symmetrically distributed within two Ag3 subunits. This induces low values of transition dipole moments as shown in Figure . This is the reason that non-linearity in this system is characterised by very low cross sections. In contrast, for the other two systems Ag15L11 and Ag31L19 situation is completely different as illustrated in Figure . There is a resonance between low lying states accessible by OPA and TPA in the spectral range around 590 nm for Ag15L11 and 730 nm for Ag31L19. Moreover, in both cases the delocalised electron distributions within the core are non-uniform as shown by the electron localisation function presented in the Figure . This is the reason for large transition dipole moments of ligand-to-metal-metal charge transfer (LMMCT) excitations involving ligands and the metal core. Notice that the increase in size of the metal core induces red shift of the resonance between OPA and TPA which is important in the context of application where the excitation wavelengths should lie within the near-IR wavelengths.
The influence of changing charge distribution from uniform to non-uniform on the values of cross section is shown for the case of Ag11L7 in Figure . The introduction of the positive external charge on the central Ag atom of the metal core induces the electron distribution from the Ag3 groups towards central part of the core as shown in the Figure . The redistribution of electron density causes the increase of transition dipole moments trough LMMCT excitations and induces an increase in TPA as shown in Figure . This means that not only size of the core, but also the structure of core responsible for redistribution of the charge might play an important role to give rise to large TPA. Therefore, this factor has to be taken into account for designing systems with large TPA cross sections. The experimental and theoretical values for the TPA cross sections are in acceptable agreement considering the simplifications introduced within the theoretical model. However, the predicted very high TPA cross sections at the wavelengths between 600 and 700 nm are not yet experimentally reachable since accessible measurements of TPA cross sections is in range of 750–900 nm. The presented results illustrate that design of NLO-phores with large TPA cross section in the NIR window can be realised according to above presented criteria forming the key concept which is of crucial importance for applications.
5. Properties of ligated cluster without metallic core
Since ligated gold clusters are particularly stable and easily preparable, the question can be raised if their powder can be prepared as material which can be directly used for medical applications. Therefore, we have investigated Au10(SG)10 nanoclusters where SG labels glutathione which have been prepared in form of powder [Citation50] as potentially new class of radiosensitisers for cancer radiotherapy. This involves the determination of NLO properties of ligated cluster with an equal Au-to-ligand ratio, thus without metallic core and therefore zero confined electrons. In the case of L = SCH3, the theoretically lowest energy structure of Au10L10 previously found [Citation82,83] belongs to catenane class containing two interpenetrating pentagons. In contrast, the structures with 8- and 12-membered rings interpenetrating each other and crown-like structure lie higher in energy. The OPA spectrum calculated using TDDFT approach for the lowest energy catenane structure is shown in Figure . The first OPA excited states are located between 400 and 300 nm and are in the good agreement with the experimental findings which show increasing intensities in this wavelengths region. The leading excitations responsible for S1 and S2 excited states shown in Figure involve Au···Au aurophilic subunit arising from penetration from two pentagons into each other that are bound to neighbouring sulphur atoms. The metal core is not formed due to the equal ratio of Au atoms and ligands but the aurophilic subunit together with neighbouring sulphur atoms forming two S-Au-S subunits is responsible for the specific electronic properties of Au10L10 NC (cf. Figures and ). Delocalization of electrons occurs within S-Au-S subunits in particularly along Au-S bonds of aurophilic Au···Au subunit. The OPA spectra obtained from the TDDFT approach for other two higher energy isomers shown in Figure differs considerably from the one obtained for the lowest energy isomer with catenane structure, particularly in the case of the crown-like structure. The comparison with the measured features confirms that the catenane structure is responsible for the experimental findings. Due to the fact that S1 state in OPA is located at around 400 nm, the lowest two-photon transition occurs approximately at 800 nm with moderate value of cross section in a good agreement with experimental findings as shown in Figure . The leading excitations within the S1 state involve Au···Au units together with neighbouring sulphur subunits. The calculations of additional TPA states gives rise to S15 located at about 600 nm with slightly higher TPA cross section but it is not experimentally accessible (cf. Figure ). However, the leading excitations involve MOs with large coefficients at gold atoms within Au···Au subunit. Resonance between OPA and TPA states has not been reached within the manifold of 20 states shown also in Figure and the values of transition dipole moments are relatively low. This allows to conclude that without the presence of the core and the delocalised electrons within the core, TPA cross sections are very low for catenane structures [Citation50].
Figure 8. TD-DFT OPA spectrum for Au10(SCH3)10 NC for the lowest energy structure belonging to the catenane class with experimental values (red line). Source: Reprinted from SI of J. Phys. Chem. Lett. 8 (9), (2017) p.1979. DOI: 10.1021/acs.jpclett.7b00611. Copyright 2017 American Chemical Society.
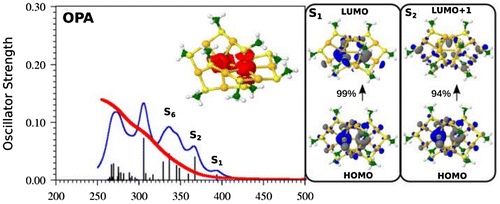
Figure 9. Side and front views of the electron localisation function (ELF) of neighbouring Sulphur atoms forming two S-Au-S subunits in which the electrons are localised. Source: Reprinted from SI of J. Phys. Chem. Lett. (2017) DOI: 10.1021/acs.jpclett.7b00611. Copyright 2017 American Chemical Society.
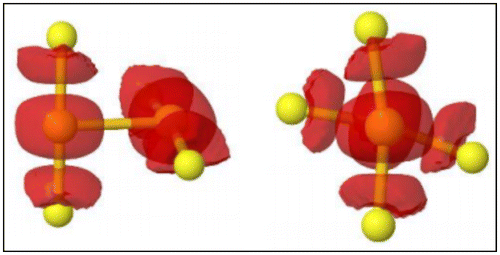
Figure 10. TD-DFT OPA spectrum for Au10(SCH3)10 NC for the two higher energy structures with experimental values (red line). Source: Reprinted from SI of J. Phys. Chem. Lett. (2017) DOI: 10.1021/acs.jpclett.7b00611. Copyright 2017 American Chemical Society.
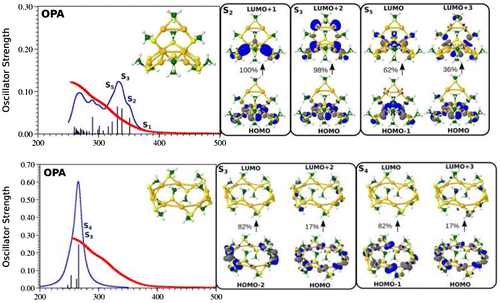
Figure 11. (top) Two-photon absorption spectrum obtained for the catenane structure of Au10(SCH3)10 with quadratic response QR-DFT method as implemented in DALTON programme. Source: Reprinted from SI of J. Phys. Chem. Lett. (2017) DOI: 10.1021/acs.jpclett.7b00611. Copyright 2017 American Chemical Society.
However, this catenane structure gives rise to characteristic circular dichroism (CD). This chirality originates from balance between the mutual interaction of the S-Au-S bonds between the two interconnected rings and chiral centres in the ligand. Moreover, the large first hyperpolarizability has been calculated for the catenane structure of Au10L10 in agreement with experimental findings [Citation50]. Although, aurophilic Au···Au subunit with neighbouring sulphur atoms in catenane structure plays a key role for NLO efficiency of Au10L10, the clusters with zero confined electrons and no core cannot give rise to large TPA cross sections.
Therefore, the design of ligated noble metal nanoclusters with structures containing metallic core with delocalised electrons giving rise to large TPA cross section serves to propose easily preparable and producible materials with adequate properties for efficient bioimaging.
6. Summary and outlook
The conceptual approach allows to design hybrid systems based on functionalised small noble metal nanoclusters taking advantage of their remarkable linear and NLO properties. Their experimental preparation and verification of proposed concepts have been successfully achieved opening potentials for medical applications. However, it remains to follow attractive future research directions for theory and experiments concerning design and preparation of hybrid systems which will allow construction of devices and production of the materials that can be directly used for medical applications.
Disclosure statement
No potential conflict of interest was reported by the author.
Funding
This work was supported by the Center of Excellence STIM at the University of Split, Croatia.
Acknowledgements
The author would like to express the gratitude to experimental group at the University of Lyon 1, prof. Michel Broyer, dr. Philippe Dugourd and dr. Rodolphe Antoine and their collaborators for long lasting fruitful cooperation as well as theoretical contributions of previous and present co-workers: prof. Roland Mitrić, dr. Alexander Kulesza, dr. Lars Gell, dr. Željka Sanader and dr. Marjan Krstić. The funding for Center of Excellence STIM at the University of Split, Croatia is also acknowledged.
References
- J. Zhao, L. Jensen, J.H. Sung, S.L. Zou, G.C. Schatz and R.P. Van Duyne, J. Am. Chem. Soc. 129 (2007) p.7647.10.1021/ja0707106
- M. Moskovits, Rev. Mod. Phys. 57 (1985) p.783.10.1103/RevModPhys.57.783
- N. Hayazawa, T. Ichimura, M. Hashimoto and Y. Inouye, J. Appl. Phys. 95 (2004) p.2676.10.1063/1.1642735
- P. Galletto, P.F. Brevet, H.H. Girault, R. Antoine and M. Broyer, J. Phys. Chem. B 103 (1999) p.8706.10.1021/jp991937t
- E. Dulkeith, A.C. Morteani, T. Niedereichholz, T.A. Klar, J. Feldmann, S.A. Levi, F.C.J.M. van Veggel, D.N. Reinhoudt, M. Moller and D.I. Gittins, Phys. Rev. Lett. 89 (2002) p.947.10.1103/PhysRevLett.89.203002
- L. Peyser-Capadona, J. Zheng, J.I. González, T.H. Lee, S.A. Patel and R.M. Dickson, Phys. Rev. Lett. 94 (2005) p.5935.10.1103/PhysRevLett.94.058301
- J. Yu, S.A. Patel and R.M. Dickson, Angew. Chem., Int. Ed. 46 (2007) p.2028.10.1002/(ISSN)1521-3773
- C.M. Ritchie, K.R. Johnsen, J.R. Kiser, Y. Antoku, R.M. Dickson and J.T.J. Petty, Phys. Chem. C 111 (2007) p.175.10.1021/jp0648487
- C.I. Richards, S. Choi, J.C. Hsiang, Y. Antoku, T. Vosch, A. Bongiorno, Y.L. Tzeng and R.M. Dickson, J. Am. Chem. Soc. 130 (2008) p.5038.10.1021/ja8005644
- V. Bonačić-Koutecký, R. Mitrić, C. Bürgel and B. Schäfer-Bung, Comput. Mater. Sci. 35 (2006) p.151.10.1016/j.commatsci.2004.12.075
- R. Antoine, T. Tabarin, M. Broyer, P. Dugourd, R. Mitrić and V. Bonačić-Koutecký, ChemPhysChem. 7 (2006) p.524.10.1002/cphc.v7:2
- I. Compagnon, T. Tabarin, R. Antoine, M. Broyer, P. Dugourd, R. Mitrić, J. Petersen and V. Bonačić-Koutecký, J. Chem. Phys. 125 (2006) p.164326.10.1063/1.2357947
- R. Mitrić, J. Petersen, A. Kulesza, V. Bonačić-Koutecký, T. Tabarin, I. Compagnon, R. Antoine, M. Broyer and P. Dugourd, J. Chem. Phys. 127 (2007) p.134301.10.1063/1.2772630
- R. Mitrić, J. Petersen, A. Kulesza, V. Bonačić-Koutecký, T. Tabarin, I. Compagnon, R. Antoine, M. Broyer and P. Dugourd, Chem. Phys. 343 (2008) p.372.10.1016/j.chemphys.2007.09.033
- T. Tabarin, R. Antoine, I. Compagnon, M. Broyer, P. Dugourd, R. Mitrić, J. Petersen and V. Bonačić-Koutecký, Eur. Phys. J. D 43 (2007) p.275.10.1140/epjd/e2007-00118-5
- T. Tabarin, A. Kulesza, R. Antoine, R. Mitrić, M. Broyer, P. Dugourd and V. Bonačić-Koutecký, Phys. Rev. Lett. 101 (2008) p.7647.10.1103/PhysRevLett.101.213001
- A. Kulesza, R. Mitrić and V. Bonačić-Koutecký, J. Phys. Chem. A 113 (2009) p.3783.10.1021/jp809118q
- A. Kulesza, R. Mitrić and V. Bonačić-Koutecký, Eur. Phys. J. D 52 (2009) p.203.10.1140/epjd/e2009-00056-2
- R. Antoine, F. Bertorelle, M. Broyer, I. Compagnon, P. Dugourd, A. Kulesza, R. Mitrić and V. Bonačić-Koutecký, Angew. Chem. Int. Ed. 48 (2009) p.7829.10.1002/anie.v48:42
- A. Kulesza, R. Mitrić and V. Bonačić-Koutecký, Chem. Phys. Lett. 501 (2011) p.211.10.1016/j.cplett.2010.11.026
- A. Kulesza, R. Mitrić, V. Bonačić-Koutecký, B. Bellina, I. Compagnon, M. Broyer, R. Antoine and P. Dugourd, Angew. Chem. Int. Ed. 50 (2011) p.878.10.1002/anie.201005419
- V. Bonačić-Koutecký, A. Kulesza, L. Gell, R. Mitrić, R. Antoine, F. Bertorelle, R. Hamouda, D. Rayane, M. Broyer, T. Tabarin and P. Dugourd, Phys. Chem. Chem. Phys. 14 (2012) p.9282.10.1039/c2cp00050d
- B. Bellina, R. Antoine, M. Broyer, L. Gell, Z. Sanader, R. Mitrić, V. Bonačić-Koutecký and P. Dugourd, Dalton Trans. 42 (2013) p.8328.10.1039/c3dt50485a
- Ž. Sanader, R. Mitrić, V. Bonačić-Koutecký, B. Bellina, R. Antoine and P. Dugourd, Phys. Chem. Chem. Phys. 16 (2014) p.1257.10.1039/C3CP52712C
- A. Kulesza, R. Mitrić and V. Bonačić-Koutecký, Phys. Chem. Chem. Phys. 14 (2012) p.9330.10.1039/c2cp23500e
- F. Bertorelle, R. Hamouda, D. Rayane, M. Broyer, R. Antoine, P. Dugourd, L. Gell, A. Kulesza, R. Mitrić and V. Bonačić-Koutecký, Nanoscale 5 (2013) p.5637.10.1039/c3nr00677h
- L. Gell, A. Kulesza, J. Petersen, M.I.S. Röhr, R. Mitrić and V. Bonačić-Koutecký, J. Phys. Chem. C 117(28) (2013) p.14824.10.1021/jp402931w
- I. Russier-Antoine, F. Bertorelle, R. Hamouda, D. Rayane, P. Dugourd, Ž. Sanader, V. Bonačić-Koutecký, P. Brevet and R. Antoine, Nanoscale 8 (2016) p.2892.10.1039/C5NR08122 J
- Ž. Sanader, M. Krstić, I. Russier-Antoine, F. Bertorelle, Ph Dugourd, P. Brevet, R. Antoine and V. Bonačić-Koutecký, Phys. Chem. Chem. Phys. 18 (2016) p.12404.10.1039/C6CP00207B
- I. Russier-Antoine, F. Bertorelle, Ž. Sanader, M. Krstić, C. Comby-Zerbino, P. Dugourd, P. Brevet, V. Bonačić-Koutecký and R. Antoine, Nanoscale 9 (2017) p.1221.10.1039/C6NR07989 J
- M.M. Alvarez, J.T. Khoury, T.G. Schaaff, M.N. Shafigullin, I. Vezmar and R.L. Whetten, J. Phys. Chem. B 101(19) (1997) p.3706.10.1021/jp962922n
- H. Häkkinen, Nat. Chem. 4 (2012) p.443.10.1038/nchem.1352
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev. 116 (2016) p.10346.10.1021/acs.chemrev.5b00703
- T. Tsukuda and H. Häkkinen, Protected Metal Clusters: From Fundamentals to Applications, Vol 9. Frontiers of Nanoscience, Elsevier Science and Technology, Dublin, 2015.
- S. Kumar, M.D. Bolan and T.P. Bigioni, J. Am. Chem. Soc. 132 (2010) p.13141.10.1021/ja105836b
- A. Desireddy, B.E. Conn, J. Guo, B. Yoon, R.N. Barnett, B.M. Monahan, K. Kirschbaum, W.P. Griffith, R.L. Whetten, U. Landman and T.P. Bigioni, Nature 501 (2013) p.399.10.1038/nature12523
- M.S. Muthu, P. Agrawal and S. Singh, Nanomedicine 11(4) (2016) p.327.10.2217/nnm.15.198
- G. Chen, I. Roy, C. Yang and P.N. Prasad, Chem. Rev. 116(5) (2016) p.2826.10.1021/acs.chemrev.5b00148
- R. Jin, Nanoscale 2(3) (2010) p.343.10.1039/B9NR00160C
- N. Goswami, K. Zheng and J. Xie, Nanoscale 6(22) (2014) p.13328.10.1039/C4NR04561 K
- W. Zhang, J. Ye, Y. Zhang, Q. Li, X. Dong, H. Jiang and X. Wang, RSC Adv. 5(78) (2015) p.63821.10.1039/C5RA11321 K
- X.-R. Song, N. Goswami, H.-H. Yang and J. Xie, Analyst 141(11) (2016) p.3126.10.1039/C6AN00773B
- M.C. Stensberg, Q. Wei, E.S. McLamore, D.M. Porterfield, A. Wei and M. Soledad Sepúlveda, Nanomedicine 6 (2011) p.879.10.2217/nnm.11.78
- X. Le Guével, C. Spies, N. Daum, G. Jung and M. Schneider, Nano Res. 5 (2012) p.379.10.1007/s12274-012-0218-1
- X.-D. Zhang, Z. Luo, J. Chen, X. Shen, S. Song, Y. Sun, S. Fan, F. Fan, D.T. Leong and J. Xie, Adv. Mater. 26(26) (2014) p.4565.10.1002/adma.v26.26
- X.L. Guevel, O. Tagit, C.E. Rodríguez, V. Trouillet, M.P. Leal and N. Hildebrandt, Nanoscale 6(14) (2014) p.8091.10.1039/C4NR01130A
- F. Aldeek, M.A.H. Muhammed, G. Palui, N. Zhan and H. Mattoussi, ACS Nano 7(3) (2013) p.2509.10.1021/nn305856t
- S.H. Yau, O. Varnavski and T. Goodson, Acc. Chem. Res. 46 (2013) p.1506.10.1021/ar300280w
- P.N. Day, R. Pachter, K.A. Nguyen and T.P. Bigioni, J. Phys. Chem. A 120(4) (2016) p.507.10.1021/acs.jpca.5b09623
- F. Bertorelle, I. Russier-Antoine, N. Calin, C. Comby-Zerbino, A. Bensalah-Ledoux, S. Guy, Ph Dugourd, P.-F. Brevet, Ž. Sanader, M. Krstić, V. Bonačić-Koutecký and R. Antoine, J. Phys. Chem. Lett. 8 (2017) p.1979.10.1021/acs.jpclett.7b00611
- M. Göppert-Mayer, Ann. Phys. 401 (1931) p.273.10.1002/(ISSN)1521-3889
- W. Kaiser and C.G.B. Garrett, Phys. Rev. Lett. 7 (1961) p.229.10.1103/PhysRevLett.7.229
- A.V. Kachynski, A. Pliss, A.N. Kuzmin, T.Y. Ohulchanskyy, A. Baev, J. Qu and P.N. Prasad, Nat. Photonics 8 (2014) p.455.10.1038/nphoton.2014.90
- W. Denk, J. Strickler and W. Webb, Science 248 (1990) p.73.10.1126/science.2321027
- C. Barsu, R. Cheaib, S. Chambert, Y. Queneau, O. Maury, D. Cottet, H. Wege, J. Douady, Y. Bretonniere and C. Andraud, Org. Biomol. Chem. 8 (2010) p.142.10.1039/B915654B
- F. Terenziani, C. Katan, E. Badaeva, S. Tretiak and M. Blanchard-Desce, Adv. Mater. 20 (2008) p.4641.10.1002/adma.v20:24
- M. Kivala and F. Diederich, Acc. Chem. Res. 42 (2009) p.235.10.1021/ar8001238
- N.H. List, R. Zaleśny, N.A. Murugan, J. Kongsted, W. Bartkowiak and H. Ågren, J. Chem. Theory Comput. 11 (2015) p.4182.10.1021/acs.jctc.5b00538
- R. Jin, Nanoscale 7 (2015) p.1549.10.1039/C4NR05794E
- J. Akola, M. Walter, R.L. Whetten, H. Häkkinen and H. Gronbeck, J. Am. Chem. Soc. 130 (2008) p.3756.10.1021/ja800594p
- M. Zhu, C.M. Aikens, F.J. Hollander, G.C. Schatz and R. Jin, J. Am. Chem. Soc. 130 (2008) p.5883.10.1021/ja801173r
- J. Xie, Y. Zheng and J.Y. Ying, J. Am. Chem. Soc. 131 (2009) p.888.10.1021/ja806804u
- J.T. Petty, J. Zheng, N.V. Hud and R.M. Dickson, J. Am. Chem. Soc. 126 (2004) p.5207.10.1021/ja031931o
- J. Zheng and R.M. Dickson, J. Am. Chem. Soc. 124 (2002) p.13982.10.1021/ja028282 l
- X. Qu, Y. Li, L. Li, Y. Wang, J. Liang and J. Liang, J. Nanomater. 2015 (2015) p.23.
- G. Ramakrishna, O. Varnavski, J. Kim, D. Lee and T. Goodson, J. Am. Chem. Soc. 130 (2008) p.5032.10.1021/ja800341v
- L. Polavarapu, M. Manna and Q.-H. Xu, Nanoscale 3 (2011) p.429.10.1039/C0NR00458H
- D. Andrae, U. Haeussermann, M. Dolg, H. Stoll and H. Preuss, Theor. Chim. Acta 77 (1990) p.123.10.1007/BF01114537
- S. Gilb, P. Weis, F. Furche, R. Ahlrichs and M.M. Kappes, J. Chem. Phys. 116 (2002) p.4094.10.1063/1.1445121
- A.D. Becke, J. Chem. Phys. 98 (1993) p.5648.10.1063/1.464913
- C. Lee, W. Yang and R.G. Parr, Phys. Rev. B:Condens. Matter 37 (1988) p.785.10.1103/PhysRevB.37.785
- P.J. Stephens, F.J. Devlin, C.F. Chabalowski and M.J. Frisch, J. Phys. Chem. 98 (1994) p.11623.10.1021/j100096a001
- T. Yanai, D.P. Tew and N.C. Handy, Chem. Phys. Lett. 393 (2004) p.51.10.1016/j.cplett.2004.06.011
- M.J.S. Dewar, E.G. Zoebisch, E.F. Healy and J.J.P. Stewart, J. Am. Chem. Soc. 107 (1985) p.3902.10.1021/ja00299a024
- N.H. List, R. Zaleśny, N.A. Murugan, J. Kongsted, W. Bartkowiak and H. Ågren, J. Chem. Theory Comput. 11 (2015) p.4182.10.1021/acs.jctc.5b00538
- P. Norman, Phys. Chem. Chem. Phys. 13 (2011) p.20519.10.1039/c1cp21951 k
- L. Frediani, Z. Rinkevicius and H. Ågren, J. Chem. Phys. 122 (2005) p.244104.10.1063/1.1944727
- K. Aidas, et al., Wiley Interdisciplinary Reviews: Computational Molecular Science 4(3) (2014) p.369.
- Dalton, A Molecular Electronic Structure Program, Release Dalton 2016. (2015. Available at https://daltonprogram.org
- P.N. Day, K.A. Nguyen and R. Pachter, J. Chem. Theory Comput. 6 (2010) p.2809.10.1021/ct100139t
- Z. Hua and L. Jensen, Chem. Sci. 8 (2017) p.4595. doi:10.1039/C7SC00968B
- K.A. Kacprzak, O. Lopez-Acevedo, H. Häkkinen and H. Grönbeck, J. Phys. Chem. C 114 (2010) p.13571.10.1021/jp1045436
- Y. Liu, Z. Tian and L. Cheng, RSC Adv. 6(6) (2016) p.4705.10.1039/C5RA22741 K
