Abstract
Containerless processing or ‘levitation’ is a valuable tool for the synthesis and characterization of materials, particularly at extreme temperatures and under non-equilibrium conditions. The method enables formation of novel glasses, amorphous phases, and metastable crystalline forms that are not easily accessed when nucleation and growth can readily occur at a container interface. Removing the container enables the use of a wide variety of process atmospheres to modify a materials structure and properties. In the past decade levitation methods, including acoustic, aerodynamic, electromagnetic, and electrostatic, have become well established sample environments at X-ray synchrotron and neutron sources. This article briefly reviews the methods and then focuses on the application of aerodynamic levitation to synthesize and study new materials. This is presented in conjunction with non-contact probes used to investigate the atomic structure and to measure the properties of materials at extreme temperatures. The use of aerodynamic levitation in research using small and wide-angle X-ray diffraction, XANES, and neutron scattering are discussed in the context of technique development. The use of the containerless methods to investigate thermophysical properties is also considered. We argue that structural motifs and in the liquid state can potentially lead to the fabrication of materials, whose properties would differ substantially from their well known crystalline forms.
Introduction
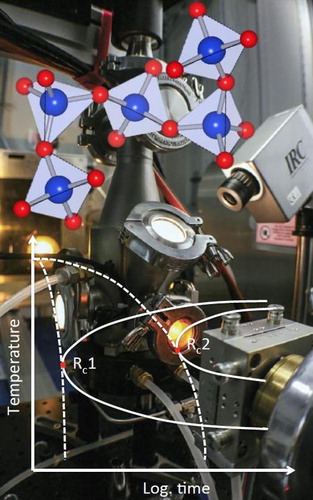
Containerless methods (also called levitation or contactless processing) represent an appealing solution to long-standing problems in materials processing [Citation1–3]. Chemical reactions between a container and its contents can lead to contamination of both components. Container walls provide extrinsic nucleation sites for crystallization and often limit the degree of supercooling that can be accomplished. Supercooling is a prerequisite for glass formation and much containerless processing work has involved the synthesis and characterization of novel glasses. The free surface of levitated drops means that drop oscillation methods can be used to investigate the fluid properties such as surface tension and the viscosity of liquids. In addition, the absence of sample holders reduces background scattering in X-ray and neutron beamline measurements. The graphic in Figure illustrates the various interactions between a sample material and its environment and the effect of enhancing the ability for supercooling and glass formation.
Figure 1. Left. The interactions between a material, its container and the surrounding atmosphere influence the behavior of a material (adapted from [Citation5]). Elimination of the container expands the range of conditions that can be accessed and avoids undesirable interactions that lead to contamination, or preclude the supercooling of molten materials. Right. Schematic time–temperature-transformation diagram illustrating the decrease in critical cooling rate for crystallization in contained (RC1) and containerless (RC2) conditions (adapted from [Citation90]).
![Figure 1. Left. The interactions between a material, its container and the surrounding atmosphere influence the behavior of a material (adapted from [Citation5]). Elimination of the container expands the range of conditions that can be accessed and avoids undesirable interactions that lead to contamination, or preclude the supercooling of molten materials. Right. Schematic time–temperature-transformation diagram illustrating the decrease in critical cooling rate for crystallization in contained (RC1) and containerless (RC2) conditions (adapted from [Citation90]).](/cms/asset/fb255c81-d10a-415e-946c-73a66e4d0961/tapx_a_1357498_f0001_oc.gif)
A supercooled liquid can be classified as ‘strong’ or ‘fragile’ depending on how fast its viscosity and relaxation times change as the glass transition temperature is approached. This can be represented as a so-called ‘Angell plot’ or in terms of a kinetic fragility index [Citation4]. For strong liquids such as SiO2, the viscosity exhibits almost Arrhenius behavior and only subtle changes in structure are observed over a very wide temperature range. Conversely, ‘fragile’ liquids such as o-terphenyl and most non-network forming oxide melts, show a fast change in their viscosity when approaching the glass transition and this behavior is attributed to locally heterogeneous dynamics as well as variations in structure. By removing the container interactions, levitation avoids extrinsic heterogenous nucleation which has a lower barrier to crystallization, enabling the study of deeply supercooled fragile liquids.
In this brief review, we primarily consider ‘steady state’ levitation for scattering and spectroscopy measurements in which the material is stably positioned at a fixed location and it can thus be probed using a variety of non-contact methods. ‘Transient’ containerless methods include drop tubes and flame or plasma torch processing [Citation5,6]. Transient techniques are valuable for the production of materials without contact, but are generally difficult to integrate with in situ characterization of the materials during processing. In a sense, the two ways of processing are somewhat complementary. Steady-state methods allow detailed investigation of materials and transient methods can then be used to manufacture materials products on a large scale.
Containerless methods are of particular value in the investigation of materials at high temperatures (>1500 °C) where reactivity with the container is a problem. This temperature range embraces scientific fields ranging from simulated geological magmas, precursor melts for functional glasses, to high temperature crystal transitions and molten nuclear fuel materials. The overall requirement for levitation is to supply a force equal to the weight of the material. For liquids, surface tension forces counteract the tendency for a drop to fragment. Several levitation methods are briefly summarized in Table . Several of these methods have previously been reviewed by Price [Citation1], Weber et al. [Citation2], Egry and Holland-Moritz [Citation3]. One of the earliest containerless techniques reported was electromagnetic levitation of metals [Citation7]. Early work on acoustic levitation was by Bucks and Mueller [Citation8] and further developed by several investigator teams [Citation9–13]. The aerodynamic levitation of solids was initially reported by Winborne et al. [Citation14] and molten samples by Coutures et al. [Citation15,16]. Potard and Grainier [Citation17] developed and used proposed gas film levitation to process ZBLAN glasses. Although issues with damage to gas diffusing membranes used to support the sample limited its applications [Citation17]. Rhim et al. [Citation18] pioneered electrostatic levitation (ESL) that has become the preferred method for work with metals and alloys [Citation19,20] and is currently used for microgravity experiments [Citation21,22]. Recent neutron and X-ray experiments using the ESL technique have been carried out by Kelton and co-workers [Citation23,24]. Various ‘hybrid’ techniques that aim to combine desirable qualities of two methods have also been developed [Citation2].
Table 1. Summary of different levitation methods.
Aerodynamic levitation
In this review, we focus on the application of aerodynamic levitation. This method has been used extensively to study metal oxides and to a lesser extent metals, alloys, and semiconductors [Citation1]. The small size of the aerodynamic levitation components enable them to be integrated with a wide variety of non-contact diagnostic methods. Because aerodynamic levitation requires a gaseous environment, it allows the use of process gases to adjust the chemistry of a sample in situ. For example, adjustment of the p(O2) using a redox mixture can be used to change the oxidation state of cations in a melt [Citation25,26]. In the case of metallic samples that may be prone to oxidation, levitation gas can be purified to parts per billion (ppb) levels of oxidizing impurities by using an oxygen getter. The use of ppb purity gas is equivalent to using a total pressure of 10−9 atmospheres and it avoids the need for use of an ultrahigh vacuum system. The presence of gas suppresses evaporation of the sample and reduces coating of the windows and other components by the evaporated material.
The essential requirement for aerodynamic levitation is that the weight of the sample (M = mass, g = gravity vector) is offset by forces from the flowing gas that can be expressed as the drag coefficient, Cd, multiplied by the integral of the gas density, ρ, and the square of its flow velocity, u, and the pressure drop over the area of sample, A, that interacts with the gas.
From this equation, it is clear that very large forces could easily be generated by flowing large volumes of gas at high velocity – as is the case in an aircraft for example. In practice, for molten samples, the size and shape of the drop is controlled by the surface tension of the liquid. Typically for molten oxides with surface tension values of a few hundred m Nm−1, the practical size limit for levitating droplets is ca. 2–4 mm in diameter.
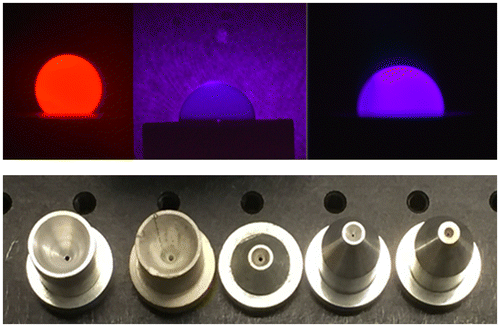
Aerodynamic levitation is typically performed using a converging–diverging nozzle with a cone semi-angle of 30–60° and an orifice at the waist of ~0.5–3 mm in diameter. Early nozzles, as reported by Coutures et al. [Citation15] were deep and the sample was only visible when viewed from above. Later work by Krishnan et al. [Citation27,28] led to development of nozzles significantly reduced in size, that enable 30–70% of the sample to be levitated above the plane of the nozzle. Nozzles have been made from aluminum alloy, stainless steel, vanadium, and non-metallic materials including boron nitride, machinable ceramic, and graphite. Aluminum alloy is a convenient choice and it provides a durable and easily cleaned nozzle material. Examples of nozzles and side views of levitated molten oxide samples are illustrated in Figure . Gas flow rates required for levitation are typically on the order of ~0.5 L/min. A variety of gases including argon, oxygen, air, and redox mixtures based on CO/CO2 have been successfully employed to levitate samples.
Figure 2. Top. Side view images of levitated molten oxide samples illuminated by incandescent emission viewed through a red filter, backlit using a blue light source and incandescent emission viewed through a blue filter. The samples shown are approximately 2.7 mm in diameter. Bottom left to right: early deep nozzles made of aluminum alloy and boron nitride, low profile nozzles that allow viewing of part of the levitated sample along the horizontal direction.
Practical issues
An important requirement for investigation of materials at high temperatures is the ability make non-contact temperature measurements on the levitated samples. Optical pyrometry is a convenient method to measure the radiance temperature of a levitated sample. The technique relies on a measurement of a portion of the thermal emission spectrum. In cases where a sample is viewed through an optical window, it is necessary to make an appropriate correction for Fresnel reflections of light from the window–atmosphere interface. Windows must be free of dust of deposits that can reduce transmission by scattering or absorption. The two windows shown in Figure illustrate the type of deposits that can form, due to material that evaporates from a sample over time. Such deposits can lead to significant errors in the temperature measurement. A second problem caused by evaporation is changes to the sample composition. In general, evaporation of multicomponent samples is incongruent, resulting in uncertainties in the composition of sample at any given stage of the measurement. Samples that evaporate significantly (more than about 5% in one minute) must be measured rapidly (i.e. in a few seconds) to minimize the composition changes.
Figure 3. Left. Photographs of a clean window and a similar window after sample material has deposited on the inner surface. The coating attenuates the emission from the hot sample leading to a lower apparent temperature being measured by an optical pyrometer. Right. A schematic plot of the apparent temperature of a sample measured at wavelengths of 0.9 and 5 μm (adapted from [Citation28]).
![Figure 3. Left. Photographs of a clean window and a similar window after sample material has deposited on the inner surface. The coating attenuates the emission from the hot sample leading to a lower apparent temperature being measured by an optical pyrometer. Right. A schematic plot of the apparent temperature of a sample measured at wavelengths of 0.9 and 5 μm (adapted from [Citation28]).](/cms/asset/7a710a42-5544-4b3f-960e-eff178a5f9a5/tapx_a_1357498_f0003_oc.gif)
Thermophysical properties
The measurement of thermophysical properties, particularly for liquids, at high temperatures is challenging due to container interactions and the influence of the container on the measurements themselves. Levitation techniques have been used in conjunction with measurements of enthalpy of fusion, density, surface tension, viscosity, and thermal conductivity [Citation1,29–35]. Much of the work on metals has been done using ESL. ESL is well suited to the requirements for measurements of density by imaging, surface tension, and viscosity by drop oscillation methods because the entire sample can be imaged. Aerodynamic levitation has been used for the measurement of density [Citation29,30,36]. The extraction of surface tension and viscosity is more difficult and has been shown for a well-known composition where the conditions for aerodynamic levitation are near ideal [Citation29,37], extension to less steadily levitated systems are under development [Citation37].
The density of levitated samples can be calculated from the measured volume of a sample of known mass. For experiments on aerodynamically levitated liquids in which only part of the sample is visible, images are analyzed with an assumption of radial symmetry around the vertical axis. The volume of the ellipsoidal drop is calculated by analysis of images of the visible portion of the sample [Citation30,31]. A dramatic example of density difference that occurs on melting BaTiO3 in an electrostatic levitator, where data on the supercooled liquid were obtained to almost 600° below the equilibrium melting point [Citation32]. The large volume expansion on melting has been confirmed using aerodynamic levitation [Citation38]. Both the solid and liquid were measured over a wide temperature range and the liquid was shown to be ~20% lower density than the cubic solid phase just below the melting point.
A drop-and-catch calorimeter has been used to measure the enthalpy of oxides at very high temperatures [Citation34,35]. The instrument uses a ‘split nozzle’ design proposed by Arai et al. [Citation39]. The nozzle can be separated along a vertical join using a fast-acting actuator. When the nozzle is opened, the hot sample falls into a calorimeter that completely encloses it to minimize heat losses and enable a measurement of the heat content. The layout is illustrated in Figure .
Figure 4. Left. Photograph of a laser-heated sample dropped into a calorimeter using the split nozzle design, reproduced from [Citation35] and published with permission of The American Ceramic Society Bulletin. Right. Photograph of a polished of homogenous glass spheroid quenched in an aerodynamic levitator.
![Figure 4. Left. Photograph of a laser-heated sample dropped into a calorimeter using the split nozzle design, reproduced from [Citation35] and published with permission of The American Ceramic Society Bulletin. Right. Photograph of a polished of homogenous glass spheroid quenched in an aerodynamic levitator.](/cms/asset/43676060-834e-4cdf-8de1-69c990e0720c/tapx_a_1357498_f0004_oc.gif)
Much of the pioneering work on aerodynamic levitation of liquids involved the measurement of the structure of oxide liquids using Nuclear Magnetic Resonance (NMR) techniques [Citation40,41]. An aerodynamic levitation nozzle was integrated into the NMR magnet to investigate the coordination of NMR-active cations as a function of temperature and process atmosphere. This early work was significantly extended by the use of radiation scattering (synchrotron X-ray and neutron) techniques to study liquids and glasses. In the following sections, several examples of the application of these techniques are presented and briefly discussed.
Radiation scattering experiments
This brings us to radiation scattering experiments, where upon heating, the thermal expansion of a material can be obtained from the refinement of the crystal structure over a wide temperature range [Citation42–44], see Figure . Microscopic determinations of thermal expansion using diffraction has several advantages. A correctly calibrated diffractometer gives a direct measurement of the crystal unit cell parameters, determined by Bragg peak positions, and an absolute measurement of density, which are unaffected by minor impurities and microstructure that may affect macroscopic measurements. Typically, high energy X-ray beams (of dimensions 0.5 mm wide × 0.2 mm high) have been used to probe the top 100–200 μm of the levitated droplet and aligned to be co-incident with the laser beam heating from above. Scattering into the bottom half of the area detector is neglected to eliminate complex attenuation corrections associated with the nozzle. Similarly, the neutron beam is collimated to probe only the top hemisphere of the levitated sample to eliminate scattering and attenuation corrections due to the nozzle.
Figure 5. Left. Data obtained on a levitated polycrystalline HfO2 sample on a spinning nozzle. Right. The rotation of the nozzle that spins the sample for an improved powder average diffraction pattern [Citation91].
![Figure 5. Left. Data obtained on a levitated polycrystalline HfO2 sample on a spinning nozzle. Right. The rotation of the nozzle that spins the sample for an improved powder average diffraction pattern [Citation91].](/cms/asset/6fcfdb62-e2ef-45f1-b659-9131f3517548/tapx_a_1357498_f0005_oc.gif)
Photons and neutrons with similar wavelengths to the spacing between atoms have long been used to probe the structure and dynamics of both ordered and disordered materials [Citation45,46]. Examples of experimental set ups of high energy X-ray diffraction, spallation neutron diffraction and XANES with an aerodynamic levitator are shown in Figure . Wide angle high-energy X-ray diffraction has been at the forefront of high-temperature aerodynamic levitation studies for the past decade. The ability to collect high-quality scattering data from the bulk sample in a few minutes or seconds over a small region of the sample has led to a variety of liquid state [Citation47,48], crystalline [Citation42,44], and glass formation studies [Citation49–52]. Comprehensive accounts of structural studies at the Japanese synchrotron, SPring-8, have been reported by Kohara et al. [Citation53], and by L. Hennet and D.L. Price et al. at the French synchrotrons, ESRF and Soleil [Citation1,49]. Greaves et al. [Citation47] have performed combined small and wide angle X-ray experiments on supercooled melts at SRS in the U.K. The phenomenon of polyamorphism in supercooled liquid yttria–alumina has sparked much debate in the literature and the reader is referred to more comprehensive texts on this subject [Citation48,54]. Benmore, Weber, Skinner and Alderman have published extensively on high energy X-ray studies of liquids and glasses at the APS in the U.S.A. [Citation5,55–58]. Wilding et al. have published on a broad range of geologically relevant melts e.g. [Citation50], while Ushakov et al. have helped pioneer high temperature crystallography using aerodynamic levitation [Citation42,43].
Figure 6. Left. Close up view of the levitation nozzle and boron nitride collimator and get lost tube in an aerodynamic levitator installed on the neutron diffractometer NOMAD [Citation92] at the Spallation Neutron Source. Top right. Photograph of a laser heated levitated droplet on the high energy X-ray beamline 6-ID-D at the Advanced Photon Source (APS). Bottom right. Diagram of the sample chamber used for XANES measurements on 20-BM-B at the APS.
![Figure 6. Left. Close up view of the levitation nozzle and boron nitride collimator and get lost tube in an aerodynamic levitator installed on the neutron diffractometer NOMAD [Citation92] at the Spallation Neutron Source. Top right. Photograph of a laser heated levitated droplet on the high energy X-ray beamline 6-ID-D at the Advanced Photon Source (APS). Bottom right. Diagram of the sample chamber used for XANES measurements on 20-BM-B at the APS.](/cms/asset/eb1a49d6-5e10-4f94-9ccf-b7f8a5983cc0/tapx_a_1357498_f0006_oc.gif)
Extensive comparisons of the atomic structures and local coordination numbers of a number of oxide melts by Kohara et al. [Citation53] and Skinner et al. [Citation59] have demonstrated relationships between structure and thermodynamic properties including density and viscosity, see Figure . One of the highest melting temperatures ever achieved in an X-ray diffraction experiment is UO2. Previously, the very high temperatures needed >3140 K and chemical reactivity of molten UO2 have prevented structural studies in simulated severe accident scenarios [Citation60]. However, the combination of laser beam heating, aerodynamic levitation, and synchrotron X-rays to obtain pair distribution function measurements of hot solid and molten UO2 has revealed a substantial increase in oxygen disorder around the lambda transition at 2670 K and a 16% drop in the average coordination number of oxygen atoms surrounding each uranium atom on melting [Citation61].
Figure 7. Left. Model three-dimensional and two-dimensional structures of several high-temperature oxides; (a) crystalline SiO2 (b) liquid SiO2 (c) liquid Al2O3 and (d) liquid ZrO2 measured using high energy X-ray diffraction combined with aerodynamic levitation [Citation53]. Right. X-ray pair distribution functions of liquid UO2 [Citation61]. The curves labeled ‘Yakub’ and ‘Refined’ represent two different molecular dynamics simulations compared to high energy X-ray pair distribution function curves on liquid UO2 (dashed). The lower dotted curves represent the atomic pair contributions from UU, UO, and OO.
![Figure 7. Left. Model three-dimensional and two-dimensional structures of several high-temperature oxides; (a) crystalline SiO2 (b) liquid SiO2 (c) liquid Al2O3 and (d) liquid ZrO2 measured using high energy X-ray diffraction combined with aerodynamic levitation [Citation53]. Right. X-ray pair distribution functions of liquid UO2 [Citation61]. The curves labeled ‘Yakub’ and ‘Refined’ represent two different molecular dynamics simulations compared to high energy X-ray pair distribution function curves on liquid UO2 (dashed). The lower dotted curves represent the atomic pair contributions from UU, UO, and OO.](/cms/asset/fc8ffe47-7484-4b0b-bf1a-b80bbeca18c7/tapx_a_1357498_f0007_oc.gif)
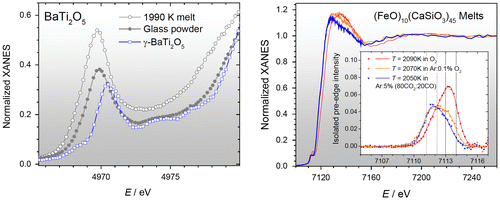
X-ray absorption near edge structure (XANES) has the advantage of being an element-specific technique capable of providing information on both formal valance and local coordination number. High-temperature aerodynamic levitation experiments using XANES are still in their infancy but experiments on liquid iron-oxide by Hennet et al. [Citation62] and molten Fe2SiO4+X using Fe K-edge XANES by Alderman et al. [Citation25] have demonstrated the feasibility (see Figure ). In the latter experiments, a large exit window perpendicular to the incoming X-ray beam was employed to measure the fluorescence signal using a Ge detector on beamline 20-BM-B at the APS. A wide range of Fe3+ contents were accessed by varying the redox potential in the levitation gas. The oxidation states were determined by analysis of the pre-edge peaks in the XANES spectra.
Figure 8. Left. Normalized XANES spectra for BaTiO5 crystal, glass and melt. Right. XANES spectra for FeO–CaSiO3 melts in different atmospheres. The insert shows isolated pre-edge features of the Fe K-edge spectra compared for three melts at different f(O2).
Other X-ray and spectroscopy techniques have been adapted to measurements under containerless conditions. The feasibility of X-ray absorption fine structure spectroscopy (EXAFS) with electromagnetic levitation has been achieved by Egry et al. (e.g. see review [Citation63]). Dynamical studies using inelastic X-ray scattering with aerodynamic levitation have been shown to provide data on phonon dispersion in hot solids [Citation64] and collective excitations in high-temperature melts [Citation65]. The Quasi Elastic Neutron Scattering (QENS) technique has been also combined with levitation techniques. Since the pioneering work of Meyer et al. [Citation66] with electromagnetic levitation, QENS has also been performed with ESL [Citation67] and the feasibility of such experiments with aerodynamic levitation has been demonstrated on calcium aluminate by Kozaily et al. [Citation68].
Despite the lower statistical accuracy of neutron diffraction data compared to X-ray, the advantages of neutrons include: (i) their sensitivity to light elements such as oxygen and (ii) the ability to exploit differences in scattering by isotopes of the same element. Since the introduction of the aerodynamic levitation technique, liquid Al2O3 has been a focus of attention [Citation69]. Such has been the interest in this material a multi-group effort has recently produced high quality neutron and X-ray diffraction data over a wide Q-range [Citation58] and combined with Reverse Monte Carlo (RMC) provided a highly constrained model of the liquid state structure. The development of high flux neutron sources has also enabled element specific neutron diffraction studies using the method of isotopic substitution [Citation70]. Here, the structural environment of the cation is probed by measuring two chemically identical samples in situ, which are the same in every respect apart from the isotopic enrichment of the cation (as illustrated in Table and Figure ).
Table 2. Faber-Ziman atom–atom pair neutron weighting factors for BaTiO3 samples containing 97% 46Ti (bcoh = 4.93 fm) and 96.2% 48Ti (bcoh = −6.08 fm), compared to those for X-ray.
Figure 9. Left. Density of solid and liquid BaTiO3 determined from images of levitated drops obtained from the equations determined in [Citation32]. Right. Neutron diffraction patterns of isotopically enriched liquid Ba46TiO3 and Ba48TiO3 at 2073 K.
![Figure 9. Left. Density of solid and liquid BaTiO3 determined from images of levitated drops obtained from the equations determined in [Citation32]. Right. Neutron diffraction patterns of isotopically enriched liquid Ba46TiO3 and Ba48TiO3 at 2073 K.](/cms/asset/378807c6-063f-4461-b617-7a041557fe1f/tapx_a_1357498_f0009_oc.gif)
In the example of barium titanate, which in the crystalline form is a widely studied ceramic with piezoelectric properties and nonlinear optics applications. The rhombohedral, orthorhombic, and tetragonal phases are all ferroelectric, and the high-temperature cubic phase system is paraelectric [Citation71]. In all these solid phases of BaTiO3, Ti forms oxygen octahedra in a classic ABO3 perovskite structure and the Ti–O and O–O distances change substantially at the phase transitions as the octahedra distort. Ti–O distances in BaTiO3 range from short 1.84–1.88 Å, to intermediate ~2.0 Å and long 2.13–2.2 Å. The shorter Ti–O distances persist in the melt and suggest a significant fraction of lower coordinate polyhedra are present in the liquid. The only previously reported occurrence of Ti4+ tetrahedrally coordinated by oxygen is in crystalline Ba2TiO4 [Citation72]. The low coordination in the melt is consistent with the observed reduced density.
Time-resolved diffraction experiments on aerodynamically levitated supercooled liquids passing through the glass transition have revealed significant structural changes. Hennet et al. [Citation49] showed the structural evolution of the fragile glass-forming liquid CaAl2O4 during supercooling from the stable liquid phase to the cold glass below Tg. The results showed a shortening of the average nearest-neighbor bond length coincided with a reduction in the average Al–O coordination number. Similarly, cooling from an ergodic high-temperature melt, Benmore et al. [Citation51] have shown a dramatic polymerization of CaO6 polyhedra in a silicate melt occurs as the non-ergodic regime is approached, starting at ~1.2 Tg as predicted from mode coupling theory. Whereas, focused laser beams can achieve very high temperatures, defocused, diffuse laser heating is required for the study of lower temperature melts, supercooled liquids and glass formation. For example, X-ray measurements of molten Na2B4O7 by Alderman et al. [Citation52] reveal a continuous structural transition from a low-density open structure toward a dense, polymerized melt close to the glass transition. The temperature-dependent nature of melt structure is found to be strongly composition-dependent, with the average B–O coordination number increasing below 1.2 Tg, as shown in Figure .
Figure 10. The average boron-oxygen coordination number, nBO, in glassy, supercooled liquid and liquid Na2B4O7. Diamonds with errorbars denote the X-ray data and the red line denotes a thermodynamic model (adapted from [Citation52]).
![Figure 10. The average boron-oxygen coordination number, nBO, in glassy, supercooled liquid and liquid Na2B4O7. Diamonds with errorbars denote the X-ray data and the red line denotes a thermodynamic model (adapted from [Citation52]).](/cms/asset/df072694-5eaa-41d0-8a0e-156841dd6870/tapx_a_1357498_f0010_oc.gif)
Containerless methods have been shown to extend glass formation by enabling deep supercooling into regions where fragile liquids start to become viscous [Citation5,73–79]. Cooling rates of the order of ~30–1000 K/s are typically obtained from quenching bulk glasses in the aerodynamic levitator. A collection of these glasses are shown in Figure .
Figure 11. Photographs of glass samples made by aerodynamic levitation Left to right: aluminum silicate containing 67 mol% Al2O3, Mg2SiO4, Er3Al5O12, Y3Al5O12, ErYLaAl5O12, and La3Al5O12 compositions. The scale along the top of the figure shows the approximate cooling rate in ºCs−1. Smaller samples have a larger surface area:volume and cool faster when the heating power is shut off. All but two of the glasses are homogeneous. The Er3Al5O12 and Y3Al5O12 composition liquids are homogeneous but undergo phase separation during cooling to form two-phase glasses.

Theory and modeling
A main benefit of high temperature X-ray and neutron scattering data on levitated samples is that it directly comparable to theory and simulations. This is especially pertinent since experimental scattering data on levitated hot samples do not provide a full description of the three-dimensional structure directly. Atomistic models can however be benchmarked against scattering data and predict both the atomic and electronic structure as well as the dynamical behavior of disordered systems. A range of modeling approaches are available, and the choice primarily depends on the question being asked. For example, both RMC [Citation80,81] and Empirical Potential Structure refinement [Citation82,83] methods provide a three-dimensional structure consistent with measured X-ray and/or neutron diffraction data, in a similar manner to a crystal structure refinement. Here, additional chemical knowledge and constraints can be added to ensure realistic atom–atom interactions. On the other hand, density functional theory (DFT, or ab initio molecular dynamics calculations) [Citation84,85] take a more theoretical approach, employing quantum mechanical calculations based on the use of functionals to predict both the atomic and electronic states of a condensed phase. Classical molecular dynamics (MD) [Citation86] simulations generally use empirical interatomic potentials to approximate the atom–atom interactions and generate the structural and dynamical evolution within a system. Both DFT and MD approaches often employ the melt-quench method for studying structural evolution from the melt to the glassy state [Citation84,87].
In some cases, multiple modeling approaches have been combined to make use of the advantages of each method. For example, an important glass former which has been the subject of several aerodynamic levitation studies over the years is the CaO–Al2O3 system, which is unusual in that it does not contain typical network-forming cations [Citation88]. Recent DFT calculations based on RMC simulations of experimental data, give cohesive energies close to the crystalline ground states and show that the 64CaO–36Al2O3 composition glass structure comprises a network of cages [Citation89]. This topologically disordered network explains the high viscosity in the melt and shows that glasses synthesized from a strongly reducing atmosphere can host solvated (trapped) electrons and bipolarons, as illustrated in Figure . Oxygen-poor environments also facilitate the study on non-oxide systems such as molten carbonates. These highly inviscid liquids are characterized by low melting points and high solubility of rare earth elements and volatile molecules. Molecular dynamics simulations show that the carbonate liquid structure is found to be heavily temperature-dependent and upon cooling a low-dimensional carbonate chain network forms. The mean chain lengths decrease as temperature is increased and as the chains become shorter the rotation of the carbonate anions becomes more rapid enhancing the diffusion of Na+ ions.
Figure 12. Left. Visualizations of a cage structure in 64CaO–36Al2O3 glass around the spin-density of the h2 cavity left by the subtracted oxygen. Al atoms (gray), Ca (green) and O (red) [Citation71]. Right. A snapshot of a molecular dynamics configuration of liquid Na2CO3 taken at T = 1100 K showing the formation of chains through the formation of bonds between C (red), and O (blue) atoms. Na atoms are shown as dots [Citation93].
![Figure 12. Left. Visualizations of a cage structure in 64CaO–36Al2O3 glass around the spin-density of the h2 cavity left by the subtracted oxygen. Al atoms (gray), Ca (green) and O (red) [Citation71]. Right. A snapshot of a molecular dynamics configuration of liquid Na2CO3 taken at T = 1100 K showing the formation of chains through the formation of bonds between C (red), and O (blue) atoms. Na atoms are shown as dots [Citation93].](/cms/asset/ef340dd3-fee4-48fa-8a68-fbd4c8eaf829/tapx_a_1357498_f0012_oc.gif)
Summary and Outlook
The early development and adoption of containerless methods was mainly driven in the 1980s by the potential for low gravity materials processing to optimize and manufacture high value added materials. As a result of support by NASA in the U.S., the European Space Agency in Europe and NASDA (now JAXA) in Japan, a variety of containerless techniques were developed. Ground-based research established effective tools for containerless processing of materials ranging from aqueous solutions to molten tungsten and refractory oxides. Along with developments in the containerless sample environment, major advances were made in analytical instrumentation, imaging, computerized data analysis, and compact high-power lasers. Very high flux, high energy X-ray synchrotrons and pulsed neutron sources became widely available for research. The combination of containerless sample environments with the new sources became a natural path to pursue. There are now well-established programs that use containerless methods at almost all major radiation scattering facilities. These capabilities are valuable for development of new functional glasses, metallic alloys, nuclear fuels, and pharmaceutical materials, research on synthetic magmas and many other areas. The ability to access states with new properties and structures will ultimately result in the design of new families of materials [Citation74]. The research community is growing and the use of containerless methods is becoming an established tool for the current generation of researchers.
The future of integrating containerless sample environments with instrumentation and expanding the range of conditions that can be accessed is very bright. Plans are being developed for high gas pressure aerodynamic levitation to increase the oxygen fugacity and enable formation of highly oxidized cations at high temperatures. Integration of optical spectroscopy instruments and high speed, high-resolution imaging to study structure, bonding, and properties in situ is a promising path. Combining small and wide angle scattering methods will allow a broad range of length scales to be probed in a single measurement. There is a resurgence of interest in using the pristine environment of low gravity to study materials and several investigator teams are currently developing containerless experiments to investigate thermophysical properties using containeless techniques on the International Space Station.
The ability to make precise measurements of structure and properties has been realized and it is still being perfected. The new capabilities are being used to develop data-sets that will feed advanced computer models to design functional materials from first principles using machine learning algorithms. The development of smart materials is underway through the Materials Genome project and Functional Glass Manufacturing Innovation Consortium in the U.S., programs at the National Institute for Materials Science in Japan and agencies in Europe and China. The goal remains to understand, predict, and control the physical properties and phase stability of materials with various types of crystalline defects at finite temperature, the liquid state at high temperatures and the recovered glassy and amorphous forms at room temperature.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported in part by the Advanced Photon Source, Argonne National Laboratory, which is funded under U.S. DOE, BES [contract number DE-AC02-06CH11357]. The work at MDI was supported by the U.S. Department of Energy (DOE) [grant number DE-SC0004604], [grant number DE-SC0007564], [grant number DE-SC0015241].
Acknowledgments
Thanks to Oliver Alderman, Lawrie Skinner, Martin Wilding, John Parise, Joerg Neuefeind, Sergey Ushakov, Alexandra Navrotsky, Shinji Kohara, Leighanne Gallington, Douglas Robinson, Anthony Tamalonis, Sam Sendelbach and our long list of collaborators and co-authors over the years for their contributions toward this project.
References
- D.L. Price, High-Temperature Levitated Materials, Cambridge, Cambridge University Press, 2010.10.1017/CBO9780511730306
- J.K.R. Weber, S. Krishnan and P.C. Nordine, J. Metals 43 (1991) p.8.
- I. Egry and D. Holland-Moritz, Eur. Phys. J. Spec. Top. 196 (2011) p.131.10.1140/epjst/e2011-01424-1
- C.A. Angell, Science 267 (1995) p.1924.10.1126/science.267.5206.1924
- J.K.R. Weber, Int. J. Appl. Glass Sci. 1 (2010) p.248.10.1111/j.2041-1294.2010.00026.x
- C.G. Levi, V. Jayaram, J.J. Valencia and R. Mehrabian, J. Mater. Res. 3 (1988) p.969.10.1557/JMR.1988.0969
- O. Muck, German Patent # 42204, Oct. 30, 1923.
- K. Bucks and H. Muller, Z. Phys. 84 (1933) p.75.10.1007/BF01330275
- E.H. Trinh, Rev. Sci. Instrum. 56 (1985) p.2059.10.1063/1.1138419
- S.E. Wolf, J. Leiterer, M. Kappl, F. Emmerling and W. Tremel, J. Am. Chem. Soc. 130 (2008) p.12342.10.1021/ja800984y
- J. Leiterer, W. Leitenberger, F. Emmerling, A.F. Thunemann and U. Panne, J. Appl. Cryst. 39 (2006) p.771.10.1107/S0021889806024915
- J.K.R. Weber, C.A. Rey, J. Neuefeind and C.J. Benmore, Rev. Sci. Instrum. 80 (2009) p.083904.10.1063/1.3196177
- C.J. Benmore, J.K.R. Weber, A.N. Tailor, B.R. Cherry, J.L. Yarger, Q. Mou, W. Weber, J. Neuefeind and S.R. Byrn, J. Pharm. Sci. 102 (2013) p.1290.10.1002/jps.23464
- D.A. Winborne, P.C. Nordine, D.E. Rosner and N.F. Marley, Metall. Trans. B 7 (1976) p.711.10.1007/BF02698607
- J.-P. Coutures, J.-C. Rifflet, D. Billard and P. Coutures, Proc. 6th European Symp. on Materials Sciences under Microgravity Conditions, Bordeaux, Dec. 2–5, (1986) p.427.
- C. Landron, X. Launay, J.C. Rifflet, P. Echegut, Y. Auger, D. Ruffier, J.P. Coutures, M. Lemonier, M. Gailhanou, M. Bessiere, D. Bazin and H. Dexpert, Nucl. Instrum. Methods Phys. Res. Sect. B, 124 (1997) p.627.10.1016/S0168-583X(97)00104-3
- J. Granier and C. Potard, 6th European Symp. on Mat. Sci. under Microgravity Conditions, ESA SP-256 (1987) p.421.
- W.K. Rhim, S.K. Chung, D. Barber, K.F. Man, G. Gutt, A. Rulison and R.E. Spjut, Rev. Sci. Instrum. 64 (1993) p.2961.10.1063/1.1144475
- T.J. Rathz, M.B. Robinson, R.W. Hyers, J.R. Rogers and D. Li, J. Mater. Sci. Lett. 21 (2002) p.301.10.1023/A:1017928022508
- P.-F. Paradis, T. Ishikawa and S. Yoda, Int. J. Thermophys. 26 (2005) p.1031.10.1007/s10765-005-6683-y
- Y.J. Kim, R. Busch, W.L. Johnson, A.J. Rulison and W.K. Rhim, Appl. Phys. Lett. 65 (1994) p.2136.10.1063/1.112768
- https://iss.jaxa.jp/en/kiboexp/pm/elf/.
- N.A. Mauro and K.F. Kelton, Rev. Sci. Instrum. 82 (2011) p.035114.10.1063/1.3554437
- N.A. Mauro, A.J. Vogt, K.S. Derendorf, M.L. Johnson, G.E. Rustan, D.G. Quirinale, A. Kreyssig, K.A. Lokshin, J.C. Neuefeind, Ke An, X.-L. Wang, A.I. Goldman, T. Egami, K. F. Kelton. Rev. Sci. Instrum. 87 (2016) p.013904.
- O.L.G. Alderman, L. Lazareva, M.C. Wilding, C.J. Benmore, S. Heald, C.E. Johnson, J.A. Johnson and H.-Y. Hah, S. Sendelbach, A. Tamalonis, L.B. Skinner, J.B. Parise and J.K.R. Weber, Geochim. Cosmochim. Acta 203 (2017) p.15.10.1016/j.gca.2016.12.038
- O.L.G. Alderman, M.C. Wilding, A. Tamalonis, S. Sendelbach, S.M. Heald, C.J. Benmore, C.E. Johnson, J.A. Johnson, H.-Y. Hah and J.K.R. Weber, Chem. Geol. 453 (2017) p.169.10.1016/j.chemgeo.2017.01.020
- S. Krishnan, J.J. Felten, J.E. Rix, J.K.R. Weber, P.C. Nordine, M.A. Beno, S. Ansell and D.L. Price, Rev. Sci. Instrum. 68 (1997) p.3512.10.1063/1.1148315
- J.K.R. Weber, C.J. Benmore, L.B. Skinner, J. Neuefeind, S.K. Tumber, G. Jennings, L.J. Santodonato, D. Jin, J. Du, and J.B. Parise, J. Non-cryst. Solids 383 (2014) p.49.
- D. Langstaff, M. Gunn, G.N. Greaves, A. Marsing and F. Kargl, Rev. Sci. Instrum. 84 (2013) p.124901.10.1063/1.4832115
- B. Glorieux, F. Millot, J.-C. Rifflet and J.-P. Coutures, Int. J. Thermophys. 20 (1999) p.1085.10.1023/A:1022650703233
- P.-F. Paradis, T. Ishikawa and S. Yoda, Meas Sci Technol 16 (2005) p.452.10.1088/0957-0233/16/2/017
- P.-F. Paradis, J. Yu, T. Ishikawa, T. Aoyama and S. Yoda, Appl. Phys. A 79(8) (2004) p.1965.10.1007/s00339-003-2133-5
- K. Ohsaka, S.K. Chung and W.K. Rhim, Acta Mater. 46 (1998) p.4535.10.1016/S1359-6454(98)00154-2
- S. Ushakov, A. Shvarev, T. Alexeev, D. Kapush and A. Navrotsky, J. Am. Ceram. Soc. 100 (2016) p.754.
- A. Navrotsky and S. Ushakov, Am. Ceram. Soc. Bull. 96(2) (2017) p.22.
- F. Kargl, C. Yuan and G.N. Greaves, Int. J. Microgravity Sci. Appl. 32 (2015) p.320212.
- M. Affatigato, Dept. Phys., Coe College, Cedar Rapids, IA, USA, private communication.
- K.-J. Lee, M.S.V. Kumar, S.-K. Jung, C.-H. Lee, C.-H. Lee and S. Yoda, J. Ceram. Proc. Res. 13(4) (2012) p.476.
- Y. Arai, P.-F. Paradis, T. Aoyama, T. Ishikawa and S. Yoda, Rev. Sci. Instrum. 74(2) (2003) p.1057.10.1063/1.1531826
- B. Coté, D. Massiot, F. Taulelle and J.-P. Coutures, Chem. Geol. 96 (1992) p.367.10.1016/0009-2541(92)90065-D
- D. Massiot, F. Fayon, V. Montouillout, N. Pellerin, J. Hiet, C. Roiland, P. Florian, J.-P. Coutures, L. Cormier and D.R. Neuville. J. Non-Cryst. Solids 354 (2008) p.249.10.1016/j.jnoncrysol.2007.06.097
- S.V. Ushakov, A. Navrotsky, J.K.R. Weber and J.C. Neuefeind, J. Am. Ceram. Soc. 1 (2015) p.1.
- P.S. Maram, S.V. Ushakov, J.K.R. Weber, C.J. Benmore and A. Navrotsky, J. Am. Ceram. Soc. 98(4) (2015) p.1292.10.1111/jace.13422
- M. Guthrie, C.J. Benmore, L.B. Skinner, O. Alderman, J.K.R. Weber, J.B. Parise and M. Williamson, J. Nucl. Mater. 479 (2016) p.19.10.1016/j.jnucmat.2016.06.042
- P.A. Egelstaff, An Introduction to the Liquid State, Oxford Series on Neutron Scattering in Condensed Matter. 2nd ed., Oxford University Press, 1992. ISBN 0-19-851012-8, 390.
- L. Hennet, V. Cristiglio, J. Kozaily, I. Pozdnyakova, H.E. Fischer, A. Bytchkov, J.W.E. Drewitt, M. Leydier, D. Thiaudière, S. Gruner, S. Brassamin, D. Zanghi, G.J. Cuello, M. Koza, S. Magazù, G.N. Greaves and D.L. Price, Eur. Phys. J. Spec. Top. 196 (2011) p.151.10.1140/epjst/e2011-01425-0
- G.N. Greaves, M.C. Wilding, S. Fearn, D. Langstaff, Q. Van Vu, F. Kargl, S. Cox, O. Majérus and C.J. Benmore, R. Weber and L. Hennet, Science 322 (2008) p.566.10.1126/science.1160766
- A.C. Barnes, L.B. Skinner, P.S. Salmon, A. Bytchkov, I. Pozdnyakova, T.O. Farmer and H. E. Fischer Phys. Rev. Lett. 106 (2011) p.119602.10.1103/PhysRevLett.106.119602
- L. Hennet, I. Pozdnyakova, A. Bytchkov, D.L. Price, G.N. Greaves, M. Wilding, S. Fearn, C.M. Martin, D. Thiaudière, J.-F. Bérar, N. Boudet and M.-L. Saboungi, J. Chem. Phys. 126 (2007) p.074906.10.1063/1.2646812
- M.C. Wilding, C.J. Benmore and J.K.R. Weber, EPL-EuroPhys. Lett. 89 (2010) p.26005.10.1209/0295-5075/89/26005
- C.J. Benmore, J.K.R. Weber, M.C. Wilding, J. Du and J.B. Parise, Phys. Rev. B 82 (2010) p.224202.10.1103/PhysRevB.82.224202
- O.L.G. Alderman, M. Liška, J. Macháček, C.J. Benmore, A. Lin, A. Tamalonis and J.K.R. Weber, J. Phys. Chem. C. 120 (2016) p.553.10.1021/acs.jpcc.5b10277
- S. Kohara, J. Akola, L. Patrikeev, M. Ropo, K. Ohara, M. Itou, A. Fujiwara, J. Yahiro, J.T. Okada, T. Ishikawa, A. Mizuno, A. Masuno, Y. Watanabe and T. Usuki, Nat. Commun. 5 (2014) p.5892.10.1038/ncomms6892
- P.F. McMillan, G.N. Greaves, M. Wilson, M.C. Wilding, D. Daisenberger, Liquid polymorphism, in Liquid polyamorphism, H. E. Stanley, ed., John Wiley & Sons, Inc., Hoboken, NJ, 2013, p.152. doi:10.1002/9781118540350.ch12.
- Q. Mei, C.J. Benmore and J.K.R. Weber, Phys. Rev. Lett. 98 (2007) p.057802.10.1103/PhysRevLett.98.057802
- C.J. Benmore. ISRN Mater. Sci., vol. 2012, Article ID 852905 19 (2012). doi:10.5402/2012/852905.
- L.B. Skinner, A.C. Barnes, P.S. Salmon, L. Hennet, H.E. Fischer, C.J. Benmore, S. Kohara, J.K.R. Weber, A. Bytchkov, M.C. Wilding, J.B. Parise, T.O. Farmer, I. Pozdnyakova, S.K. Tumber and K. Ohara, Phys. Rev. B 87 (2013) p.024201.10.1103/PhysRevB.87.024201
- O.L.G. Alderman, L.B. Skinner, C.J. Benmore, A. Tamalonis and J.K.R. Weber, Phys. Rev. B. 90 (2014) p.094204.10.1103/PhysRevB.90.094204
- L.B. Skinner, C.J. Benmore, J.K.R. Weber, J. Du, J. Neuefeind, S.K. Tumber and J.B. Parise, Phys. Rev. Lett. 112 (2014) p.157801.10.1103/PhysRevLett.112.157801
- A. Navrotsky, Science 346 (2014) p.916.10.1126/science.aaa0163
- L.B. Skinner, C.J. Benmore, J.K.R. Weber, M. Williamson, A. Tamalonis, A. Hebden, T. Wiencek, O.L.G. Alderman, M. Guthrie, L. Leibowitz and J.B. Parise, Science 346 (2014) p.984.10.1126/science.1259709
- L. Hennet, C. Landron, P. Berthet, J.-P. Coutures, T. Jenkins, C. Aletru and N. Greaves, Jpn. J. Appl. Phys. 38 (1999) p.115.10.7567/JJAPS.38S1.115
- I. Egry and D. Holland-Moritz, Eur. Phys. J. Spec. Top. 196 (2011) p.131.10.1140/epjst/e2011-01424-1
- A. Alatas, A.H. Said and H. Sinn, J. Phys. Chem. Solids 66 (2005) p.2230.10.1016/j.jpcs.2005.09.077
- A.H. Said, H. Sinn, A. Alatas, C.A. Burns, D.L. Price, M.-L. Saboungi and W. Schirmacher, Phys. Rev. B 74 (2006) p.172202.10.1103/PhysRevB.74.172202
- A. Meyer, S. Stuber, D. Holland-Moritz, O. Heinen and T. Unruh, Phys. Rev. B 77 (2008) p.092201.10.1103/PhysRevB.77.092201
- T. Kordel, D. Holland-Moritz, F. Yang, J. Peters, T. Unruh, T. Hansen and A. Meyer, Phys. Rev. B 83 (2011) p.104205.10.1103/PhysRevB.83.104205
- J. Kozaily, L. Hennet, H.E. Fischer, M. Koza, S. Brassamin, S. Magazù and F. Kargl, Phys. Stat. Sol. C 8 (2011) p.3155.10.1002/pssc.201000753
- C. Landron, L. Hennet, T.E. Jenkins, G.N. Greaves, J.P. Coutures and A.K. Soper, Phys. Rev. Lett. 86 (2001) p.4839.10.1103/PhysRevLett.86.4839
- L. Skinner, C.J. Benmore, J.K.R. Weber, S. Tumber, L. Lazareva, J. Neuefeind, L. Santodonato, J. Du and J.B. Parise, J. Phys. Chem. B 116(45) (2012) p.13439.10.1021/jp3066019
- G.H. Kwei, A.C. Lawson, S.L.G. Billinge and S.-W. Cheong, J. Phys. Chem. 97 (1993) p.2368.10.1021/j100112a043
- B.L. Dubey and A.R. West, Nat. Phys. Sci. 235 (1972) p.155.10.1038/physci235155a0
- S. Kohara, K. Suzuya, K. Takeuchi, C.-K. Loong, M. Grimsditch, J.K.R. Weber, J.A. Tangeman and T.S. Key, Science 303 (2014) p.1649.
- P.F. McMillan, Nat. Mater. 7 (2008) p.843.10.1038/nmat2313
- P. Kidkhunthod, A. Bootchanont and A.C. Barnes, J. Non-Cryst. Solids 448 (2016) p.27.10.1016/j.jnoncrysol.2016.06.034
- L.B. Skinner, A.C. Barnes, P.S. Salmon, H.E. Fischer, J.W.E. Drewitt and V. Honkimaki, Phys. Rev. B 85 (2012) p.064201.10.1103/PhysRevB.85.064201
- J.K.R. Weber, A.D. Hixson, J.G. Abadie, P.C. Nordine and G.A. Jerman, J. Am. Ceram. Soc. 83 (2000) p.1868.
- J.K.R. Weber, S. Sen, R.E. Youngman, R.T. Hart and C.J. Benmore, J. Phys. Chem. B 112 (2008) p.16726.10.1021/jp807964u
- J.A. Tangeman, B.L. Phillips, A. Navrotsky, J.K.R. Weber, A.D. Hixson and T.S. Key, Geophys. Res. Lett. 28 (2001) p.2517.10.1029/2000GL012222
- R.L. McGreevy and L. Pusztai, Mol. Simul. 1 (1988) p.359.10.1080/08927028808080958
- R.L. McGreevy, J. Phys.: Condens. Matter. 13 (2001) p.R877.
- A.K. Soper, Phys. Rev. B 72 (2007) p.104204.
- A. K. Soper, J. Phys.: Condens. Matter. 19 (2007) p.33.
- J. Sarnthein, A. Pasquarello and R. Car, Phys. Rev. B 52 (1995) p.12690.10.1103/PhysRevB.52.12690
- J. Hafner, J. Comput. Chem. 29 (2008) p.2044.10.1002/jcc.v29:13
- M.P. Allen and D.J. Tildesley, Computer Simulation of Liquids, Clarendon Press, Oxford, 1987.
- B.P. Feuston and S.H. Garofalini, J. Chem. Phys. 89 (1988) p.5818.10.1063/1.455531
- L. Hennet, I. Pozdnyakova, V. Cristiglio, S. Krishnan, A. Bytchkov, F. Albergamo, G.J. Cuello J.-F. Brun, H/.E. Fischer, D. Zanghi, S. Brassamin, M.-L. Saboungi and D.L. Price, J. Non-Cryst. Solids 353 (2007) p.1705.10.1016/j.jnoncrysol.2007.01.040
- J. Akola, S. Kohara, K. Ohara, A. Fujiwara, Y. Watanabe, A. Masuno, T. Usuki, T. Kubo, A. Nakahira, K. Nitta, T. Uruga, J.K.R. Weber and C.J. Benmore, Proc. Natl. Acad. Sci. USA 110(25) (2013) p.10129.10.1073/pnas.1300908110
- W.F. Gale and T.C. Totemeier (eds.), Smithells Metals Reference Book, 8th ed., Elsevier, Amsterdam, 2004.
- J.K.R. Weber, A. Tamalonis, C.J. Benmore, O.L.G. Alderman, S. Sendelbach, A. Hebden and M.A. Williamson, Rev. Sci. Instrum. 87 (2016) p.073902.10.1063/1.4955210
- J. Neuefeind, M. Feygenson, J. Carruth, R. Hoffmann and K. Chipley, Nucl. Instrum. Meth. B 287 (2012) p.68.10.1016/j.nimb.2012.05.037
- M.C. Wilding, M. Wilson, O.L.G. Alderman, C.J. Benmore, J.K.R. Weber, J.B. Parise, A. Tamalonis and L. Skinner, Sci. Rep. 6 (2016) p.24415.10.1038/srep24415
