Abstract
The ocelot (Leopardus pardalis) is one of the most widespread neotropical felids but data on its distribution and population status in several countries are scarce. Here, we present estimates of density for lowland forest of eastern Ecuador. We used camera trap data and capture–recapture analyses to estimate ocelot density within a local area (~650 ha) within Yasuní Biosphere Reserve, recognized as a globally important area for biodiversity conservation. We estimated densities for dry and wet seasons (~2200 trap days total) using CAPTURE and both half and full values of the mean maximum distance moved (MMDM), as recommended for estimating densities. Estimated densities for the dry season were higher than during the wet season (dry: 41–74 ind/100 km2; wet: 32–52 ind/100 km2). Ocelots were captured more frequently at night than during the day and some individuals were captured more consistently in areas close to the Tiputini River. Density estimates for ocelots in the Yasuní region are somewhat higher than in other neotropical areas, but similar to other sites in the Amazon region. Based on the number of ocelots recorded in this relatively small study area, it is clear that the region is an important area for conservation. Further studies that take into account more complex estimates such as survival rates and migration as well as differences in growth and availability of resources could provide more evidence for the importance of this region.
El ocelote (Leopardus pardalis) es uno de los felinos neotropicales mas estudiados, pero datos sobre su distribución y estado de sus poblaciones son escasos en varios países. Aquí presentamos estimaciones de densidad para Ecuador. Se utilizaron datos de cámaras-trampa y análisis de captura-recaptura para estimar la densidad ocelotes dentro de un área (~ 650 ha) de bosques de tierras bajas del este de Ecuador, dentro de la Reserva de Biosfera Yasuní, reconocida como un área de importancia mundial para la conservación de la biodiversidad. Estimamos densidades para épocas seca y de lluvias (~2.200 trampa días en total) utilizando CAPTURE y tanto la mitad como los valores completos de la media máxima distancia recorrida (MMDM). Las densidades estimadas para la estación seca fueron más altas que durante la estación húmeda (seca: 41–74 ind/100 km2; lluvias: 32–52 ind/100 km2). Los individuos de ocelotes fueron capturados con mayor frecuencia durante la noche y algunos de manera más consistente en las zonas cercanas al río Tiputini. Las estimaciones de densidad de ocelotes en la región de Yasuní son mas altas comparadas con otras regiones del Neotrópico y similares a estimaciones previas de otros sitios en la región amazónica. Con base en el número de ocelotes registrados en el área de estudio, es evidente que la región es un área importante para la conservación. Nuevos estudios que tengan en cuenta estimaciones más complejas podrían proporcionar evidencia más sólida de la importancia de esta región.
Introduction
Felids are important components of most neotropical forests and larger species such as the jaguar (Panthera onca) and puma (Puma concolor) have been well studied; however, less attention has been paid to smaller felids.[Citation1] The ocelot, Leopardus pardalis, is a medium-sized cat with an extensive geographical range, from southern Texas to Argentina.[Citation2] Ocelots are found in a variety of habitats including grassland, thorn shrub and tropical rainforest.[Citation3] In the tropics, ocelots generally are the most abundant felid and densities can be high.[Citation4] Compared to jaguars and pumas, ocelots have smaller home ranges, which vary depending on available prey (smaller in areas with more resources) and latitude (larger at higher latitudes).[Citation2] Consequently, in the highly productive Amazon Basin, ocelot populations are generally denser and individuals have smaller home ranges. In Ecuador, ocelots are found along the Pacific coast lowlands, western Amazonia and the foothills of the Andes. They inhabit both wet and dry tropical and subtropical forests, between 0 and 1500 m. They are found almost exclusively in primary or old second-growth forests.[Citation5]
Although once threatened by extensive poaching, populations have partially recovered after legislation banned hunting of the species in the 1980s.[Citation2] Currently, although ocelots are threatened by loss of habitat because of forest fragmentation, populations have shown some resilience to human disturbances.[Citation6] The IUCN Red List classifies the ocelot as ‘least concern’ because of its abundance and high genetic diversity.[Citation7]
Using direct methods (e.g. observations) to study ocelots in lowland tropical forests is challenging because of their mostly nocturnal, solitary and secretive habits.[Citation1] Since they have become available, camera traps have provided a method to study rarely seen wildlife and allow analysis of activity patterns, sex ratios and population estimation based on the traditional method of mark-recapture for species, such as ocelots, that are individually recognizable. Camera traps also provide an efficient method for studying ocelots because they, and other felids, often prefer to walk on man-made trails.[Citation8] Consequently, when cameras are placed on trails, the rate of capture is increased beyond a random model. Camera traps have been used to study ocelots in Bolivia,[Citation1] Brazil,[Citation9] Argentina,[Citation2] Perú,[Citation6] the United States[Citation3] and Ecuador.[Citation10] The goal of our study was to provide much-needed estimates of density for ocelots in eastern Ecuador. Because ocelot density is highest at low latitudes and elevations, we expected that, because of its location, ocelot density would be high at Tiputini Biodiversity Station in the lowlands of eastern Ecuador.
Methods
Study area
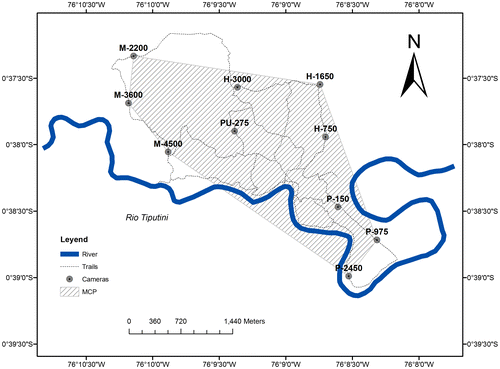
We conducted our research at Tiputini Biodiversity Station (TBS), Orellana Province, Ecuador (ca. 0°38′S, 76°10′W, 190–270 m elevation). TBS was founded in 1994 by the Universidad San Francisco de Quito (USFQ) on a tract of undisturbed lowland rainforest adjacent to Yasuní National Park and within Yasuní Biosphere Reserve, one of the most biologically diverse regions of the world.[Citation11] The reserve, composed of Yasuní National Park, the contiguous Waorani Ethnic Reserve and a buffer zone, encompasses an area of approximately 20,000 km2 [Citation12] and is home to indigenous groups such as the Waorani and the Kichwa, many of whom depend on the forest for much or all of their subsistence. The station and nearby areas contain a variety of habitats including terra firme and varzea forest, palm swamps and other wetlands, as well as areas of succession that follow treefalls, wind throws or other natural disturbances. The mean annual precipitation is ca. 2800 mm. There is a wet season from April to early August, when >65% of the annual rain falls (monthly average ca. 385 mm); 15% occurs from November to February (monthly average ca. 140 mm; based on data from 1998 to 2002 [Citation13]). January often is particularly dry and March, August, September and October are considered transition months.[Citation14]
Camera trapping
We operated camera traps during two 16-week surveys, from November 2010 through February 2011 (dry season) and from April through July 2011 (wet season). We used digital camera traps (CuddeBack Capture® Model 1125) triggered by an infrared motion and heat detector to document the occurrence of ocelots (and other large terrestrial mammals and birds; see [Citation14–16]). We deployed cameras at 10 stations located approximately 1–1.2 km apart along narrow (generally <1 m wide) preexisting trails within terra firme and varzea forest (Figure ) (see [Citation15] for further description). Each station consisted of paired cameras opposing each other, at a height of 30–40 cm from the ground, to photograph both flanks of the animal. No bait was used to attract animals at any station. Cameras remained continuously activated during the time of the survey (except when batteries failed or other malfunctions occurred); date and time were automatically stamped on each photograph. Cameras were set with a minimum time between photographs of 1 min and were checked at regular intervals to replace batteries, memory cards and to ensure proper functioning. The arrangement of the camera traps reflected the original focus of our study, which was to evaluate diversity and abundance of large terrestrial mammals and birds.
Analysis
We summarized images by individual, hour and date. We used a combination of distinguishing characters, including the patterns of rosettes, spots and stripes on flanks as suggested by Trolle and Kery [Citation17] to identify individual ocelots. Males were identified by the presence of testes and females by the lack thereof. In some cases, we were unable to positively identify an individual and those pictures were excluded from the analysis. We compiled camera trap data into 16 one-week periods per season and considered each week to be a separate sampling occasion. Multiple photographs of the same individual during the same sampling period were considered to be a single record.
Most studies on density of felines have traditionally used CAPTURE [Citation18,19,20] to calculate the population size and then to estimate the density by dividing estimated abundance by the estimated effective trapping area (ETA). CAPTURE has been widely used in previous analyses of camera trap data [Citation21] and, therefore, it allows us to compare our results with those of previous studies. CAPTURE tests observed data against several capture–recapture models, and recommends the model that best fits the data.[Citation22] Models are ranked by CAPTURE ranging from 0 to 1, with 1 indicating best fit.[Citation2] The abundance estimate was then used to derive an estimate of density (D), defined as D = N/A, where N is animal abundance and A is the area sampled. To determine the area effectively sampled, we calculated a core area as the minimum convex polygon defined by all outer trap stations (as suggested by Karanth and Nichols [Citation23]). Given that the actual area used by ocelots is not simply the area defined by this polygon, a buffer was added to the area defined by the outer traps, so that the sampling area extended beyond the outer polygon limits.[Citation18,23,24] An overestimate of density would occur if the outer trap polygon area was used without a buffer.[Citation25] We used ArcGIS™ 9.3 and the extension Hawth’s Analysis Tools (spatialecology.com/htools) to draw a minimum convex polygon, calculate the areas of buffers and to estimate the distance between camera stations where ocelots were photographed.
We estimated the width of the buffer by obtaining the mean maximum distance moved (MMDM): the mean of the maximum linear distance moved from one trap to another [Citation26] across all individuals photographed at two or more locations during the sampling period. This was used to estimate home-range diameter.[Citation22,24] Although this approach has proven to be robust in simulation studies and is commonly used, there is debate about a proper buffer value (i.e. MMDM vs. ½MMDM).[Citation26,27] Trolle and Kéry [Citation9] concluded that one-half of MMDM would be an underestimate of the actual buffer and that the actual value should lie between ½MMDM and MMDM (i.e. when telemetry data are not available to provide a more detailed estimate). On the other hand, Parmenter et al. [Citation28] compared estimates of boundary strips based on different methods and found that the use of the full MMDM to calculate effective trapping area provided the most accurate density estimate.[Citation25] Here, we report density estimates for dry and wet seasons using both ½ MMDM and full MMDM.
Results
The total camera trap effort was ~2200 trap-nights. During the dry season (Nov–Feb), 28 images of eight individual ocelots were obtained from seven camera locations. From those, three photographs could not be identified to individual and were not considered for the analysis. CAPTURE suggested the jackknife estimator (Mh) and we used that model to estimate ocelot population size.[Citation26] Mh assumes heterogeneity in capture probability among individuals, but that the probability of each individual being recaptured remains the same throughout the sampling period.[Citation25] Such heterogeneity among individuals reflects the fact that activity and ranging behavior likely vary with sex, age and social status [Citation30] and that individual ocelots will differ in their likelihood of encountering different camera stations.[Citation23,24,31] Based on Mh the estimated population during dry season was 11 (SE ± 2.5) (Table ). Estimated density varied from 41.5 ± 9.2 to 74 ± 16.6 individuals per every 100 km2 (Table ).
Table 1. Population estimates provided by CAPTURE for the two seasons using model Mh (jackknife estimator).
Table 2. Effective areas sampled using Half and Full MMDM to estimate the buffer and density estimates for each season.
During the wet season, we obtained 14 images of six individuals from seven sites. CAPTURE estimated an abundance of six individuals based on the jackknife estimator (Mh) (Table ). Densities varied from 32.7 ± 4.9 to 52.3 ± 7.8 ind/100 km2 (Table ).
Nine individual ocelots were photographed during both survey periods combined. Five of eight individuals captured during the dry season were recaptured in the wet season; one new individual was recorded in the wet season. Males outnumbered females both in the dry season (six males, three females) and wet season (four males, two females). Multiple individuals were photographed in different locations, but one individual male had the highest number of captures in both seasons and at the same locations (12 captures, seven in P-150, three in P-975 and two in P-2450), suggesting that it is a permanent resident in that part of the study area and that it repeatedly used the trail as a travel route. Other individuals, in contrast, were photographed only once in each season and at different locations at considerable linear distances apart (>2 km). The fact that individuals were recaptured after several months does, however, suggest that they are also permanent residents of the area.
Discussion
Density estimates for ocelots at TBS were somewhat higher than in some other Neotropical areas [Citation1,2,9,32,33] and temperate regions [Citation3] (Table ). When using ½MMDM as a buffer, our estimates were similar to those obtained by Emmons [Citation8] in the Peruvian Amazon (80 ocelots/100 km2) and by Trolle and Kéry [Citation17] in the Pantanal of Brazil (56 ocelots/100 km2); the former study was based on telemetry, whereas the second used camera trap data. Salvador and Espinosa [Citation10] worked in two areas near the site of the current study and estimated densities of 84.5 and 93.3 ocelots/100 km2 using ½MMDM and ¼MMDM, respectively. However, the camera array in that study was designed to estimate abundance for jaguars and, as a consequence, used distances between camera stations of 2–3 km. Those distances were too large to adequately estimate ocelot movements and most likely exceeded the home range of individual ocelots resulting in few recaptures among trap locations. There are still some drawbacks and possible biases related to the use of MMDM which have been discussed elsewhere.[Citation24,29] Clearly, buffer width is a critical factor in deriving density estimates and in our study the MMDM was the largest source of variation in density estimation. Dillon and Kelly [Citation26] and Maffei and Noss [Citation22] suggest that, independent of the density estimation, it is important to obtain information on home range size in order to determine the appropriate camera spacing and buffer size, in order to adapt the camera traps to the local studied populations.
Table 3. Estimated ocelot density (ind/100 km2) in various habitats, author and methodology used.
Camera traps and radio telemetry have been used in many previous studies to estimate density and activity of ocelots in different ecosystems within its range [Citation1–3,8, 9,34] (Table ). However, it is still not clear what factors have the strongest influence on ocelot abundance.[Citation31] It has been suggested that ocelot density might increase with increasing precipitation and that rainfall and latitude are correlated with the abundance of ocelots across their continental range.[Citation1,31] This relationship could be explained as a result of increased prey densities with increased productivity [Citation31,35] abundance of prey is a limiting factor for the abundance of most wild cat populations.[Citation24,36]
Our density estimates were somewhat lower in the wet season than in the dry season. Large-scale patterns in rainfall and latitude likely reflect larger-scale patterns of productivity overall, but would not be expected to influence seasonal patterns of activity at a local site. Lower daily rainfall and temperatures may cause ocelots and prey species to increase their activity during drier months. This might reflect changes in behavior (higher activity), changes in habitats used (travel more widely along trails) or more movement in areas sampled by cameras, thereby elevating trap success rates.[Citation15] However, although capture success could increase, density should remain the same.
Most studies on ocelots have covered larger areas of forest than the present study. Given the relatively small area covered in this study (26.4 km2) ocelot abundance can be considered remarkably high in this region of Yasuní. Due to the geographic location of TBS, this high number could be explained by latitudinal gradients in primary productivity (greater productivity at lower latitudes) [Citation31] abundance of prey and lack of hunting pressure.
Methodological issues, such as determining the correct effective area of action of ocelots, also may have important implications for density estimates. When buffers are extended around the outer perimeter of trap stations, it is possible that the total area might encompass some areas that are not suitable for ocelots. In such cases, where there are areas that are not likely to contain ocelots (e.g. towns, lakes, rivers), those areas should be subtracted from the effective sampling area.[Citation37] In the present study, because some camera stations were located in close proximity to the Tiputini River (Figure ), the outer polygon of trap locations, augmented by the buffer (either based on ½MMDM or full MMDM), included areas across the river (south side), which were considered part of the area surveyed in the calculation of density. Since the habitat on the south side of the river is suitable for ocelots and ocelots are known to occur there (DM, pers. obs.), we did not subtract this area for the calculations. Doing so would have reduced the effective area sampled and increased the estimates of densities.
In a simultaneous study on jaguars at TBS [Citation16] cameras located along the peninsular area of the station, close to the river (P-150, P-975, P-2450, Figure ) experienced the lowest capture rates of jaguars. In contrast, in this study, the same camera locations produced the highest capture rates for ocelots. Similarly, in a previous study on monthly activity patterns of some terrestrial mammals at TBS [Citation15] a decline in ocelot activity coincided with a sharp increase in activity of jaguars. Although ocelots occur in sympatry with other cats like jaguars, pumas and jaguarondis (Herpailurus yagouaroundi), these results suggest some level of avoidance of jaguars by ocelots. The high photographic capture rate of ocelots in this area near the river (three males and two females) might also suggest that this specific area has higher prey availability for ocelots and hence is a more desired habitat; the hypothesis that ocelots, in general, prefer habitats in close proximity to a river has not been confirmed. It is also possible that differences in capture rates between locations could be attributed to seasonal differences in ocelot activity patterns.
In this study, as in a previous one [Citation15,38] ocelots were the most photographed and the most nocturnal of all species of cats recorded, with pronounced peaks in activity around 22:00. These results are similar to those reported for ocelots in other studies [Citation2,8,39] where ocelots were more active at night but still showed some level of activity during daylight hours. This activity pattern could be influenced by availability and activity of prey, since preferred prey of ocelots also have nocturnal habits (e.g. Proechimys, Myoprocta and other small rodents).[Citation32,40] Alternatively, ocelot activity also may reflect an adaptation to avoid competition with or predation by larger cats.[Citation41]
Conservation implications
Wild cats are particularly vulnerable in Ecuador given the large number of existing environmental and sociocultural conflicts. Accurate estimates of animal densities are vital for comparative analyses across different ecosystems in order to focus efforts on management and conservation and the ocelot may constitute a good model to understand what factors affect the abundance of Neotropical felids.[Citation31] Considering the geographic location of TBS, this is particularly important because Yasuní has been recognized as the most biologically diverse region in all Amazonia.[Citation11] The area of TBS is largely unaffected by human activities, as it is relatively distant from extractive activities (i.e. oil) and experiences very low levels of hunting. Because of its nearly pristine condition, the availability of food, water, breeding areas and shelter for protection is high for most species. However, large areas of the park are also subject to human disturbances, such as oil exploration and extraction, agriculture and illegal logging, that are thought to negatively influence wildlife.[Citation11] Although ocelots are not part of the diet of indigenous groups and ocelots and people do not compete for the same prey [Citation10] they are killed for other reasons (poultry predation, mistaken beliefs, senseless killings DM, pers. obs). Uncontrolled hunting of wild animals, mainly peccaries (Tayassu pecari and Peccari tajacu), pacas (Cuniculus paca) and woolly monkeys (Lagothrix poeppigii) also occurs extensively along oil access roads in the area, particularly via Maxus [Citation42] with little control by park rangers or governmental authorities. Although oil companies control access to the road by outsiders, little or nothing is done to stop colonization by indigenous groups, which has been growing rapidly in recent years (DM, pers. Obs). Thus, ocelots may face a combination of threats from direct killing, reductions in prey availability and habitat loss associated with increases in human activities.[Citation31]
This study followed methods used in many previous studies of wild felids,[Citation25] but caution is required when taking conservation and management decisions. Overestimation of density could lead to underestimates of threats faced by endangered felid species and could hence slow the implementation of conservation strategies. Given the substantial increase in the number of studies using camera traps for density estimation, particularly for felids, standardization of techniques is vital for comparative analyses, both across sites and within sites across years.[Citation26] Although this study provides estimates of ocelot densities in the Ecuadorian Amazon, further studies are needed that explore such issues as survival rates, movement patterns, growth and availability of resources in this and other areas in Ecuador. Studies at additional sites across Yasuní would allow an assessment of ocelot populations over larger areas and over longer time periods, allowing identification of priority areas for conservation. Large geographic areas within the Amazon can experience substantial and rapid deforestation and other human-caused impacts at very fast speeds, which make the lack of solid information an obstacle to the implementation of appropriate conservation strategies. From the conservation perspective and due to the tremendous lack of data on felines in Ecuador, it is also vital to promote more detailed studies that can provide further evidence of the extraordinary productivity of the area. Studies at further sites across Yasuní will also allow to assess and monitor ocelot populations over time, identifying areas with conservation priority.
Author contributions
DM and JGB did the field work, analyzed the data and wrote the first draft of the manuscript. KS and DR reviewed and improved the manuscript.
Acknowledgments
We thank the many staff and volunteers who helped check cameras during this study, particularly, Charlotte Arthun, Juanpablo Muñoz, Eduardo Gutierrez, Mariano Grefa, Froilán Macanilla and Jaime Guerra. We also appreciate the help of Consuelo de Romo in facilitating our work at Tiputini and the many staff that made working there such a pleasure. Work at Tiputini Biodiversity Station was conducted in accordance with research permit No. 0005-FAU-MAE-DPOPNY, Ministerio de Ambiente, Ecuador.
Associate Editor: Esteban Suárez.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
Support for this study was provided by National Geographic Society [7602-04], Universidad San Francisco de Quito, Tiputini Biodiversity Station, University of Missouri – St. Louis, University of Florida and Walton Expeditions.
References
- Maffei L, Noss AJ, Cuéllar E, et al. Ocelot (Felis pardalis) population densities, activity, and ranging behaviour in the dry forests of eastern Bolivia: data from camera trapping. J. Trop. Eco. 1999;21:349–353.10.1017/S0266467405002397
- Di Bitetti MS, Paviolo A, De Angelo C. Density, habitat use and activity patterns of ocelots (Leopardus pardalis) in the Atlantic Forest of Misiones, Argentina. J. Zoo. (London). 2006;270:153–163.
- Haines AM, Janecka JE, Tewes ME, et al. The importance of private lands for ocelot Leopardus pardalis conservation in the United States. Oryx. 2006;40:1–5.
- Emmons LH, Feer F. Neotropical rainforest mammals: a field guide. Chicago, IL: University of Chicago Press; 1997.
- Tirira D. Guía de Campo de los Mamíferos del Ecuador [Field Guide to the Mammals of Ecuador]. Quito: Ediciones Murciélago Blanco; 2007.
- Kolowski JM, Alonso A. Density and activity patterns of ocelots (Leopardus pardalis) in northern Peru and the impact of oil exploration activities. Biol. Conserv. 2010;143:917–925.10.1016/j.biocon.2009.12.039
- Caso A, Lopez-Gonzalez C, Payan E, et al. Leopardus pardalis. In: IUCN 2010. IUCN red list of threatened species. Version 2010.1. Available from: www.iucnredlist.org; 2008.
- Emmons LH. A field study of ocelots (Felis pardalis) in Peru. Revue d’Ecologie (la Terre et la Vie). 1988;43:133–157.
- Trolle M, Kery M. Camera-trap study of ocelot and other secretive mammals in the northern Pantanal. Mammalia. 2005;69:405–412.
- Salvador J, Espinosa S. Density and activity patterns of ocelot populations in Yasuní National Park, Ecuador. Mammalia. 2015. ISSN (Online) 1864–1547, ISSN (Print) 0025–1461, doi:10.1515/mammalia-2014-0172.
- Bass MS, Finer M, Jenkins CN, et al. Global conservation significance of Ecuador's Yasuní National Park. PLoS ONE. 2010;5:e8767.10.1371/journal.pone.0008767
- Finer M, Vija V, Ponce F, et al. Ecuador’s Yasuni biosphere reserve: a brief modern history and conservation challenges. Environ. Res. Lett. 2009;4:1–15.
- Karubian J, Fabara J, Yunes D, et al. Temporal and spatial patterns of macaw abundance in the Ecuadorian Amazon. Condor. 2005;107:617–626.10.1650/0010-5422(2005)107[0617:TASPOM]2.0.CO;2
- Blake JG, Mosquera D, Guerra J, et al. New locality records and the first photographs of living Echimys saturnus (dark tree rat, Echimyidae) from eastern Ecuador. Ecotropica. 2010;16:141–144.
- Blake JG, Mosquera D, Loiselle B, et al. Temporal activity patterns of terrestrial mammals in lowland rainforest of eastern Ecuador. Ecotropica. 2012;18:137–146.
- Blake JG, Mosquera D, Guerra J, et al. Yasuní – a hotspot for jaguars Panthera onca (Carnivora: Felidae)? Camera-traps and jaguar activity at Tiputini Biodiversity Station, Ecuador. Rev. Biol. Trop. 2014;62:689–698.10.15517/rbt.v62i2.11115
- Trolle M, Kéry M. Estimation of ocelot density in the Pantanal using capture–recapture analysis of camera-trapping data. J. Mammal. 2003;84:607–614.10.1644/1545-1542(2003)084<0607:EOODIT>2.0.CO;2
- Otis D, Burnham K, White G, Anderson D. Statistical inference from the capture data on closed animal populations. Wildlife Monogr. 1978;62:1–135.
- Rexstad E, Burnham, K. User’s guide for interactive program CAPTURE abundance estimation of closed population animal populations. Colorado: Colorado State University; 1991.
- Noss AJ, Gardner B, Maffei L, et al. Comparison of density estimation methods for mammal populations with camera traps in the Kaa-Iya del Gran Chaco landscape. Anim. Conserv. 2012;15:527–535.10.1111/j.1469-1795.2012.00545.x
- Tobler M, Powell W. Estimating jaguar densities with camera traps: problems with current designs and recommendations for future studies. Biol. Conserv. 2013;159:109–118.10.1016/j.biocon.2012.12.009
- Maffei L, Noss AJ. How small is too small? Camera trap survey areas and density estimates for ocelots in the Bolivian Chaco. Biotropica. 2008;40:71–75.
- Karanth U, Nichols JD. Estimation of tiger densities in India using photographic captures and recaptures. Ecology. 1998;79:2852–2862.10.1890/0012-9658(1998)079[2852:EOTDII]2.0.CO;2
- Karanth U, Nichols JD. Monitoring tigers and their prey: a manual for researchers, managers and conservationists in Tropical Asia. Bangalore: Centre for Wildlife Studies; 2002.
- Soisalo M, Cavalcanti S. Estimating the density of a jaguar population in the Brazilian Pantanal using camera-traps and capture–recapture sampling in combination with GPS radio-telemetry. Biol. Conserv. 2006;129:487–496.10.1016/j.biocon.2005.11.023
- Dillon A, Kelly M. Ocelot home range, overlap and density: comparing radio telemetry with camera trapping. J. Zool. 2008;275:391–398.10.1111/jzo.2008.275.issue-4
- Wilson KR, Anderson DR. Evaluation of two density estimators of small mammal population size. J. Mammal. 1985;66:13–21.10.2307/1380951
- Parmenter RR, Yates TL, Anderson DR, et al. Small-mammal density estimation: a field comparison of grid-based vs. web-based density estimators. Ecol. Monogr. 2003;73:1–26.10.1890/0012-9615(2003)073[0001:SMDEAF]2.0.CO;2
- Soisalo, MK. Estimating jaguar (Panthera onca) densities using camera-trap capture–recapture surveys in a southern Pantanal ranch, Brazil [ masters dissertation]. Wildlife Research Group, University of Cambridge, 2004.
- Harmsen BJ, Foster RJ, Doncaster CP. Heterogeneous capture rates in low density populations and consequences for capture-recapture analysis of camera-trap data. Popul. Ecol. 2010;53:253–259. doi:10.1007/s10144-010-0211-z.
- Di Bitetti MS, Paviolo A, De Angelo C, et al. Local and continental correlates of the abundance of a Neotropical cat, the ocelot (Leopardus pardalis). J. Trop. Ecol. 2008;24:189–200.
- Dillon A, Kelly M. Ocelot (Leopardus pardalis) in Belize: the impact of trap spacing and distance moved on density estimates. Oryx. 2007;41:469–477.
- Ludlow M, Sunquist M. Ecology and behavior of ocelots in Venezuela. Nat. Geog. Res. 1987;3:447–461.
- Crawshaw PG. Comparative ecology of ocelot (Felis pardalis) and jaguar (Panthera onca) in a protected subtropical forest in Brazil and Argentina [ PhD dissertation]. Gainesville (FL): University of Florida; 1995.
- Herfindal I, Linnell JDC, Odden J, et al. Prey density, environmental productivity and home-range size in the Eurasian lynx (Lynx lynx). J. Zool. 2005;265:63–71.10.1017/S0952836904006053
- Carbone C, Gittleman JL. A common rule for the scaling of carnivore density. Science. 2002;295:2273–2276.10.1126/science.1067994
- Silver SC, Ostro LET, Marsh LK, et al. The use of camera traps for estimating jaguar Panthera onca abundance and density using capture/recapture analysis. Oryx. 2004;38:148–154.
- Blake JG, Mosquera D, Loiselle B, et al. Spatial and temporal activity patterns of ocelots Leopardus pardalis in lowland forest of eastern Ecuador. J. Mammal. 2015; 97:455–463. doi: 10.1093/jmammal/gyv190
- Kasper CB, Mazim FD, Soares JBG, et al. Composicao e abundancia relative dos mamiferos de medio e grande porte no Parque Estadual do Turvo, Rio Grande do Sul [Composition and relative abundance of large and medium sized mammals of Parque Estadual do Turvo, Rio Grande do Sul]. Brasil. Rev. Brasil Zool. 2007;24:1087–1100.10.1590/S0101-81752007000400028
- Emmons LH. Comparative feeding ecology of felids in a neotropical rainforest. Behav. Ecol. Sociobiol. 1987;20:271–283.10.1007/BF00292180
- Vilas Boas Goulart F, Graipel ME, Tortato MA, et al. Ecology of the ocelot (Leopardus pardalis) in the atlantic forest of Southern Brazil. Neotrop. Biol. Conserv. 2009;4:137–143.
- Suárez E, Morales M, Cueva R, et al. Oil industry, wild meat trade, and roads: indirect effects of oil extraction activities in a protected area in Northeastern Ecuador. Anim. Conserv. 2009;12:364–373.10.1111/acv.2009.12.issue-4