Abstract
For the first time in Ecuador, a defecation rate is calculated from three captured páramo rabbit Sylvilagus brasiliensis andinus individuals. Combining this defecation rate, the pellet count method, and Novaro et al.'s (1992) hare density equation, páramo rabbit densities were calculated in four páramo localities with different vegetation structures. A correlation was found between the páramos' vegetation diversity and páramo rabbit densities. This study shows that pellet counting is an economical and non-invasive method that permits the estimation of páramo rabbit abundances in páramo ecosystems. Páramo rabbit abundances may serve as a tool to measure the effects of human activity over small mammal population sizes. Further studies of small páramo mammals will help better understand páramo ecosystem functions and will provide insights into its land management and conservation.
Con la captura de tres conejos de páramo, Sylvilagus brasiliensis andinus, se calculó la tasa de defecación para esta especie por primera vez en Ecuador. Combinando la tasa de defecación estimada, el método de conteo de bolitas de heces de conejos y la ecuación de densidad de liebres de Novaro et al. (1992 ) las densidades de conejo de páramo se calcularon en cuatro páramos con distintas estructuras de vegetación. Los resultados sugieren que existe una correlación entre la diversidad de la vegetación de estos páramos y su densidad de conejos de páramo. Este estudio comprueba que el conteo de bolitas de heces de conejo de páramo es un método no invasivo y económico que permite la estimación de la abundancia de conejos de páramo. La abundancia de conejos de páramo puede servir como una herramienta para medir los efectos de la actividad humana sobre los tamaños de poblaciones de mamíferos pequeños de este ecosistema. Futuros estudios científicos sobre los pequeños mamíferos del páramo ayudarán a comprender mejor el funcionamiento del páramo para su manejo y conservación.
Introduction
The páramo, a high-altitude ecosystem of Northern South America, forms a unique environment found in the Andes of Venezuela, Colombia, Ecuador and northern Peru,[Citation1,2] between the upper limits of montane forests (3000–3500 m) and the lower limits of perpetual snow.[Citation3] For a long time, páramo ecosystems have been recognized and studied because of their critical role in the regulation and supply of water for human consumption and a diverse range of society needs including irrigation, electricity generation and sanitation. Simultaneously, recent studies are shedding light on the biogeographic origins, diversity and endemism patterns of the unique vegetation that characterizes these environments. Despite these advances, many aspects of the structure and functioning of these ecosystems remain unexplored. For example, there is still little information on the structure and dynamics of wildlife populations, and there is virtually no data on their temporal patterns and their interactions with páramo vegetation. This gap is especially important because páramo ecosystems with little human disturbance are very sensitive and can harbor a complex wildlife community, including mountain tapirs (Tapirus pinchaque) Andean bears (Tremarctos ornatus), mountain lions (Puma concolor), páramo rabbits (Sylvilagus brasiliensis andinus) and a diverse guild of raptors, whose role and interactions with the dynamics of the ecosystem has not been researched enough.
Current information about páramo mammals is limited to a few studies mostly related to the distribution, diet and natural history of rare and large species such as the spectacled bear, the mountain tapir and the white-tailed deer Odocoileus virginianus.[Citation4–8] In addition to these studies, some research has been done on the diversity, abundance and distribution of small mammals and birds in specific páramo localities.[Citation9–14] In sharp contrast, there is virtually no information about the status of key mammal species in the páramo and the changes that their populations could be experiencing as a result of increasing pressure from anthropogenic activities. Among these species, the páramo rabbit S. brasiliensis andinus, a sub species of the cottontail forest rabbit S. brasiliensis [Citation15] and is of special interest as it probably plays a critical role in structuring páramo communities, both as a pervasive herbivore consuming a wide variety of plant species, but also as an important food source for several predators such as the Andean fox Lycalopex culpaeus, the long-tailed weasel Mustela frenata, the mountain lion P. concolor and the black-chested buzzard eagle Geranoaetus melanoleucus.[Citation16–18] From this perspective, the study of the páramo rabbit is likely to offer relevant information evaluating the impacts of human activities on the resource-base of the ecosystem (plant community structure) and also on the higher levels of the trophic webs. In addition, it will be increasingly important as managers, landowners and decision-makers struggle to find integrative approaches to manage and conserve páramo ecosystems in the context of the complex matrix of the human intervention that they experience.
Knowledge about the cottontail forest rabbit is mostly limited to several studies concerning its occurrence and its role in the diet of carnivore species in savanna and tropical forest sites in the lowlands of Eastern South America.[Citation19–23] In contrast, the high-altitude sub-species that occurs in the northern Andes has received little attention and our knowledge is limited to a short report on its diet [Citation15] and a three-year study conducted in the páramo of Mucubaji in the Venezuelan Andes.[Citation24] The latter study described several aspects of the reproductive biology of the species and population densities of 2.2–3.9 individual/ha.[Citation24] Besides these studies, little is known about the biology of this species in other parts of its Andean distribution, and the natural or anthropogenic factors that could influence the temporal and spatial variation in its population numbers. Moreover, to the extent of our knowledge, no information exists on the potential relationships that could exist between habitat structure and the population size of this rabbit.
The northeastern Andes of Ecuador offers an excellent opportunity to explore the factors that could influence the population status and behavior of key mammal species such as the páramo rabbit. This region exhibits a wide range of vegetation types that are related to local environmental conditions and to varying levels of human activities from localities with little or no human intervention to areas that have been heavily impacted. In this context, the main objectives of this study were (i) to estimate population densities of the páramo rabbit in the northeastern páramos of Ecuador and (ii) to assess if páramo rabbit abundances and behavior vary among páramo localities with different vegetation structures. In the long-term, we hope that the results of this study will be useful in future studies on the ecology of páramo animal communities and also in the monitoring of restoration or conservation programs in this ecosystem.
Site descriptions
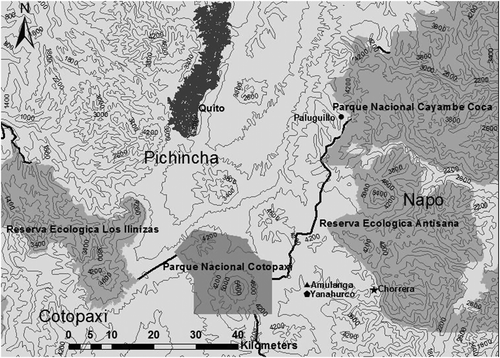
Between December 2012 and July 2013, we studied four páramo sites standing along Ecuador’s northeastern Andes (Figure ). These sites were selected to represent a range of different vegetation types that are roughly associated with a gradient of human disturbance. These páramo sites range in altitude from 3600 to 3980 m. At 3980 m, the Paluguillo private reserve is characterized as a moderately impacted páramo area surrounded by private farms. Paluguillo is fragmented by a secondary dirt road that connects to a major highway less than 1 km away. Hunting is not allowed at this site although control is sporadic and some infrequent hunting could take place. In addition, feral dogs have been observed roaming in this territory. The Yanahurco site, standing at an altitude of 3600 m, is located in the Yanahurco Private Reserve (YPR), a 26,000 ha reserve of protected páramo located in the buffer zone of Cotopaxi National Park. This páramo is considered a moderately impacted páramo due to the presence of cattle and invasive wild horses that occur in low densities. Human presence is frequent since the reserve receives hundreds of local and international tourists, but hunting is banned and the presence of feral dogs has not been observed. Our third site (Amulanga) is also part of the YPR and is located at an altitude of 3980 m, 5 km away from the Yanahurco site. Human presence at Amulanga is very scarce since access to this site is possible only by hiking or horseback riding. Due to its location, Amulanga showed fewer signs of human disturbance than Paluguillo and Yanahurco, although signs of previous fires that have consumed the vegetation are still visible in specific areas. In this location, wild horses and cattle are present in very low densities, hunting has been strictly banned and no evidence of feral dogs has been noted. Finally, our fourth site was Chorrera located at the outskirts of the YPR. The most isolated site, at an altitude of 3600 m, was selected to represent a páramo with very low levels of human disturbance. The Chorrera site is reachable only after traveling 14 km from the Yanahurco cabins. Hunting is also banned and no signs of burning or feral dogs have been observed.
Methods
In order to characterize the structure of the plant community we used a combination of primary and secondary information. First, in each site we randomly established between one and six 50-m long transects. Fewer transects were established in Amulanga and Chorrera due to the presence of a more homogenous vegetation structure and the presence of harsh climatic conditions that made field work extremely difficult. All transects were located in areas with gentle slopes (<30°), in altitudinal ranges between 3600 and 3980 m and were at least 100 m apart from each other. Along each transect, plant life-forms [Citation25] and percent coverage of each plant life-form was recorded. From this information, we derived parameters such as percentage of bare ground coverage and Shannon diversity index for plant life-forms. Additionally, secondary information concerning the ease of human access to each site by road was noted.
In order to estimate the densities of páramo rabbits we used a pellet-count method.[Citation26,27] Although direct rabbit observation during line transect sampling or live trapping could be used to estimate páramo rabbit density because it provides absolute numbers, these methods are time-consuming and rabbit densities cannot be estimated when abundances are low.[Citation28,29] In addition, live trapping is invasive as the method involves direct manipulation of the animals.[Citation29] Thus, a less intensive and less invasive method such as counting pellets can be used to estimate rabbit densities.[Citation28,29] This method is based on (i) the estimation of defecation rates of the páramo rabbit and (ii) direct counts of páramo rabbit pellets in different field locations over a standard period of time. Pellet counts are widely used to monitor rabbits and other lagomorphs [Citation28] and have been shown to provide information on habitats with varying rabbit abundances.
In order to estimate defecation rates, this study required the capture and short-term confinement of a few páramo rabbits. Capture efforts were carried out in Paluguillo and Yanahurco due to easy access. Páramo rabbits were captured with Tomahawk live-traps following Barnett’s [Citation10] trapping procedure. Bait consisted of a mixture of bananas, apples, peanut butter, oatmeal, alfalfa, lettuce, carrots and apple cider. Captured rabbits were penned individually in a 1 × 2 m holding pen. The pen was closed off with metal fencing on all lateral sides (buried 50 cm into the ground) and the top to prevent rabbit escape and predator infiltration. The floor of the pen consisted of natural páramo covered with vegetation of known páramo rabbit consumption such as Poaceae and Cyperaceae.[Citation15]
Each individual rabbit was confined for one day. Before entering its pen, the floor was checked to assure that all previous rabbit pellets and other animal feces had been removed. Water was provided at all times and the natural floor vegetation acted as shelter and food for the rabbit during its brief stay. Captured rabbits were weighed, observed and released. Further manipulation of these individuals was avoided in order to prevent stress and death. Following each rabbit’s confinement, the individuals were released back into the same location they were originally captured from. Immediately after the rabbit’s release, its pellets were collected and quantified. A defecation rate was calculated for the individual using its body weight, time penned and number of pellets produced. Individual defecation rates were combined to obtain an average defecation rate for páramo rabbits that was utilized in the second part of this study. Several studies have utilized this confinement procedure [Citation29–32] in order to calculate rabbit defecation rates since calculating defecation rates utilizing free roaming individuals in the wild is logistically difficult and expensive.
Pellet counts were carried out along six 50-m long transects randomly established at least 100 m apart from each other. Along each transect, circular plots (1 m in diameter) were established at 10 m intervals (six plots per transect). Plots were not placed on páramo rabbit latrines, which can be clearly distinguished from the extremely high density of pellets of different relative ages. The center of each plot was permanently marked with a flagged pole in order to easily identify plots during revisits. Sites were sampled during 5–8 months. Sampling effort varied at sites due to variable driving distances, available field work days for researchers and logistical needs among study sites.
During the first visit to each site, all existing rabbit feces were removed from the pellet count plots. One month later, the plots in each study site were revisited and the new rabbit pellets in each plot were quantified. Transects were revisited monthly for a duration of 5–8 months per site. Rabbit abundances were estimated with the equation developed by Novaro et al. [Citation26].
where D = rabbit abundance (indiv./ha), X = mean number of pellets per plot, T = time between plot pellet clearing and pellet count (days), R = defecation rate (number of pellets dropped per animal per day) and A = area of each plot (m2). Finally, a mean páramo rabbit density was calculated for each location by grouping the rabbit densities calculated per transect. Differences in the mean densities among locations were tested with Kruskal–Wallis non-parametric test, followed by Dunn’s pairwise comparisons. Linear correlations were analyzed between páramo rabbit densities and varying diversity of plant forms. All statistical analyses were carried out with software JMP 9.0.1.
Results
All our study sites were dominated by tussock grasses (Calamagrostis sp.), which mean percent cover ranged between 45% ± 23 (mean ± standard error) and 73%. Yanahurco was the site with the highest diversity of plant life-forms (Shannon diversity index; H = 0.53) and included not only a high cover of tussock grasses (61% ± 12), but also an important layer of shrubs and erect herbs, which covered 6 and 5% of the ground, respectively. The site with the second-highest diversity of plant life-forms was Paluguillo (H = 0.42) where shrubs and mosses covered between 8 and 4% of the ground, respectively. The remaining two sites had lower diversity of plant life-forms (Amulanga: H = 0.25; Chorrera: H = 0.35), which reflects a simpler vegetation structure with only two or three life-forms as compared to the seven found in Yanahurco and Paluguillo (Table ).
Table 1. Mean percentages of plant cover by various vegetation life-forms and bare ground in four páramo sites in northeastern Ecuador.
The total trapping effort was 100 trap nights consisting of 90 trap nights at Paluguillo and 10 trap nights at Yanahurco. Trapping efforts were carried out only in these two study sites since live-trap transportation was facilitated by the access of roads at both sites. Three rabbits were captured (N = 3 rabbits) and confined for 24 h each. All capture individuals were sub-adult individuals in good body condition. The average páramo rabbit defecation rate was 280.3 ± 35 pellets/individual/day (mean ± standard error).
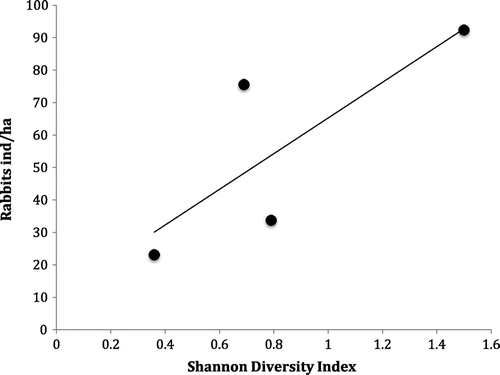
Mean rabbit densities ranged from 23 to 92 indiv./m2 and were significantly different between sites (χ2 = 21.7, p < 0.0001; Figure ). Yanahurco had the highest calculated páramo rabbit abundance 92 ± 25 indiv./ha (mean ± standard error) followed by Paluguillo (76 ± 18 indiv./ha). Páramo rabbit densities at these sites were between two and four times higher than corresponding values at the (Chorrera 34 ± 17 indiv./ha) and Amulanga (23 ± 6 indiv./ha) sites (Figure ). In all study sites, the estimated páramo rabbit densities tended to increase during summer months (June and July), especially in Paluguillo, Chorrera and Yanahurco where the densities of rabbits from June to July were between two and four times higher than densities between December and February (Table ). Although our number of sites was low, we found a strong and significant correlation (non-parametric Spearman’s correlation: ρ = 0.95; p < 0.001) between the mean density of páramo rabbits and the Shannon index of diversity of plant life forms at each site (Figure ). According to this analysis, the highest densities of páramo rabbits were found in the sites with higher diversity of plant life-forms.
Figure 2. Mean rabbit density (rabbits/ha) and standard error in four study sites calculated with Novaro et al.’s [Citation26] hare density formula. N = number of months pellet count took place (Amulanga N = 5, Paluguillo N = 8, Chorrera N = 7 and Yanahurco N = 7). Sites that do not share a letter were significantly different (p < 0.05) according to Dunn’s non-parametric test of pairwise comparisons.
![Figure 2. Mean rabbit density (rabbits/ha) and standard error in four study sites calculated with Novaro et al.’s [Citation26] hare density formula. N = number of months pellet count took place (Amulanga N = 5, Paluguillo N = 8, Chorrera N = 7 and Yanahurco N = 7). Sites that do not share a letter were significantly different (p < 0.05) according to Dunn’s non-parametric test of pairwise comparisons.](/cms/asset/830b7cd6-62e6-43fc-891d-ceeb3a509aa5/tneo_a_1179846_f0002_b.gif)
Table 2. Monthly density estimates (rabbits/ha) of the rabbit Sylvilagus brasiliensis andinus at four locations of northeastern Ecuador.
Discussion
The main objective of this study was to estimate the densities of S. brasiliensis andinus in páramo localities with different plant community structures in northeastern Ecuador. The mean páramo rabbit densities at our study sites ranged from 23 to 92 indiv./ha and our calculated densities were similar to densities estimated for Oryctolagus cuniculus in southern Italy (26–95 indiv./ha; [Citation33]). In the latter study, rain was negatively correlated with the density of rabbits. Similarly, in our study sites, rabbit densities peaked during the summer near the end of the sampling period (June and July of 2013). Other studies have also reported significant changes in rabbit densities probably related to seasonal changes in rabbit activity. For example, Novaro et al. [Citation26] reported higher European hare (Lepus capensis) densities during summer months. However, this pattern is unlikely to explain the contrasting densities that we report here, as the seasonality at our study sites is not comparable to that of temperate areas.
Our páramo rabbit densities are higher than those documented for other leporid species throughout the world, including densities of 0.85–2.7 indiv./ha for O. cuniculus in Iberian Mediterranean habitats.[Citation34] Our estimate is also much higher than the only other density reported in the literature for S. brasiliensis (2.2–3.9 indiv./ha) in the high Andes of Venezuela.[Citation24] Although some of these differences could be attributed to the field methods used in different studies, previous research has shown that density estimates generated through pellet counts and live trapping methods are closely correlated. For example, Forys and Humphrey [Citation29] concluded that both live trapping and pellet counting were accurate and effective methods for estimating marsh rabbit (Sylvilagus palustris hefneri) densities since they were significantly correlated and both offered an accurate estimate of the density calculated from radio-tracking data. However, the previous study does mention that a defecation rate calculated with captive rabbits could be a possible source of error since rabbits may defecate more when they are not captive. In this context, the wide range of densities that we report in this study probably reflects important differences in vegetation structures between sampling sites that result in significant differences in the population dynamics of S. brasiliensis.
This investigation’s páramo rabbit densities of 23–92 indiv./ha are somewhat similar to densities estimated for the zacatuche rabbit (Romerolagus diazi) of 0–174 rabbits/ha in Mexico.[Citation35] In this previous study, rabbit populations were also studied at sites with varying vegetation structures and the highest population density was found at a site with a vegetation structure of Muhlenbergia macroura and Festuca tolucensis which the zacatuche rabbit uses for food and shelter.[Citation35] In our study, the mean density of páramo rabbits was highly correlated to the diversity of plant life-forms (i.e. the complexity of the vegetation) in each site. Although we do not have specific field information to explain this pattern, we speculate that the higher rabbit density in areas that have heterogeneous vegetation could be explained by at least two main factors. First, more complex vegetation could offer better protection against predators such as the Andean Fox (L. culpaeus), the black-chested buzzard eagle (G. melanoleucus), or the long-tailed weasel (M. frenata) that are common in the northeastern Ecuadorian Andes. Such patterns have been previously reported for O. cuniculus, a species that exhibited higher abundances at sites with more woodland, as compared with sites where grass, scrub and crops were dominant.[Citation36] Second, the higher diversity of the vegetation could result in a more reliable and diverse food source for the rabbits. For example, Valero and Durant [Citation15] suggest that the páramo rabbit may be an opportunistic species that eats 3 to 12 vegetation species abundantly each month in addition to eating other available plants. Regarding this possibility, several studies have shown that one of the main impacts of burning and grazing is the simplification of vegetation structure and, more specifically, the elimination or reduction of the shrub layer in the plant community.[Citation25,37–39] From this perspective, the lower abundances of páramo rabbits in sites where vegetation structure was less diverse could reflect the cumulative effect of the lower availability of food sources and hiding places, and the direct impacts that fires might have on the population dynamics of this species. Additionally, there is anecdotal evidence that there may be seven year cycles for the páramo rabbit which could account for the differences we observed across sites. The distinction between the influences of all these factors clearly deserves further attention. Moreover, it is likely that large population fluctuations in páramo rabbit densities could have cascading impacts throughout the food web because of their roles as primary consumers, seed dispersers and as a food source to a wide assemblage of predators and scavengers. Despite their potential importance, the nature of these interactions remains largely unexplored for the Tropical Andes.
Our study reports the first population density estimates for S. brasiliensis andinus for the high Andes of Ecuador, and the first attempt at exploring the factors that could control the large temporal and spatial variability of its population numbers. This survey suggests that the population densities of S. brasiliensis andinus are strongly associated with the structure of the páramo vegetation, although further studies are needed to establish the nature and prevalence of this relationship. Furthermore, our data strongly suggest that long-term sampling is urgently needed to understand the temporal cycles of the populations of the páramo rabbit, and their ecological causes and consequences in páramo ecosystems.
Author contributions
JG and ES contributed extensively to the work presented in this paper. All authors discussed the results and implications and commented on the manuscript at all stages.
Associate Editor: Miguel Pinto.
Disclosure statement
No potential conflict of interest was reported by the author.
Funding
This investigation was self-funded by JG (MSc student).
Acknowledgments
We would like to express our gratitude to Stella de la Torre who provided insight and expertise that greatly contributed to this investigation. We are grateful to USFQ’s (Universidad San Francisco de Quito) COCIBA (Colegio de Ciencias Biológicas y Ambientales) for providing the Tomahawk live-traps used in this investigation. We thank Miguel Pinto, Will Cresswell, and one anonymous reviewer for providing valuable comments that improved the content of this article. We extend our warmest thanks to friends and colleagues that helped in the fieldwork despite extreme weather conditions, and our special appreciation to Fernando Cobo, owner of Yanahurco.
References
- Sklenář P, Luteyn JL, Ulloa CU, et al. Flora genérica de los Páramos: guía ilustrada de las plantas vasculares [Generic flora of the Páramos: illustrated guide of vascular plants]. Vol. 92, Memoirs of the New York Botanical Garden. New York: The New York Botanical Garden Press; 2005.
- Mena P, Hofstede R. Los Paramos Ecuatorianos. Botánica Económica de los Andes Centrales [Ecuadorian Páramos. Economical Botany of the central Andes]. La Paz: Universidad Mayor de San Andrés; 2006; p. 91–109.
- Hofstede R, Segarra P, Mena P, editors. Los Páramos del Mundo [Páramos of the World]. Quito: Proyecto Atlas Mundial de los Páramos. Global Peatland Initiative/NC-IUCN/EcoCiencia; 2003.
- Suarez L. Seasonal distribution and food habits of spectacled Bears Tremarctos ornatus in the highlands of Ecuador. Stud. Neotrop. Fauna Environ. 1988;23:133–136.10.1080/01650528809360755
- Downer CC. Observations on the diet and habitat of the mountain tapir (Tapirus pinchaque). J. Zool. 2001;254:279–291.10.1017/S0952836901000796
- Lizcano DJ, Pizarro V, Cavelier J, et al. Geographic distribution and population size of the mountain tapir (Tapirus pinchaque) in Colombia. J. Biogeog. 2002;29:7–15.10.1046/j.1365-2699.2002.00654.x
- Kattan G, Hernandez OL, Goldstein I, et al. Range fragmentation in the spectacled bear Tremarctos ornatus in the northern Andes. Oryx. 2004;38:155–163.
- Albuja L. Biología y ecología del venado de cola blanca (Odocoileus virginianus, Ustus Gray, 1874) en los páramos de Oyacahi y Antisana, Ecuador [Biology and ecology of the white tailed deer (Odocoileus virginianus, Ustus Gray, 1874) in the páramos of Oyacachi and Antisana, Ecuador]. Politécnica. 2007;27:34–57.
- Vuilleumier F. Biogeography of high Andean birds, essay on various aspects of their evolution. Anales del Instituto de la Patagonia Serie Ciencias Naturales. 1992;21:39–66.
- Barnett A. Small mammals of the Cajas plateau, Southern Ecuador: Ecology and natural history. Bull. Florida Nat. Museum Nat. History. 1999;42:161–217.
- Cresswell W, Hughes M, Mellanby R, et al. Densities and habitat preferences of Andean cloud-forest birds in pristine and degraded habitats in north-eastern Ecuador. Bird Conserv. Int. 1999;9:129–145.10.1017/S0959270900002252
- Koenen M, Koenen S. Effects of fire on birds in páramo habitat of northern Ecuador. Ornitol. Neotrop. 2000;11:155–163.
- Woodman N. A new species of small-eared shrew from Colombia and Venezuela (Mammalia: Soricomorpha: Soricidae: Genus Cryptotis). Proc. Biol. Soc. Washington. 2002;115:249–272.
- Bonaccorso E. Avifauna of a high Andean forest: Bosque protector Cashca Totoras, Bolivar province, Ecuador. Ornitol. Neotrop. 2004;15:483–492.
- Valero L, Durant P. Análisis de la Dieta del Conejo de Paramo, Sylvilagus brasiliensis meridensis Thomas, 1904 (Lagomorpha: Leporidae) En Mucubaji, Merida, Venezuela [Diet analysis of the páramo rabbit, Sylvilagus brasiliensis meridensis Thomas, 1904 (Lagomorpha: Leporidae) in Mucubaji, Merida, Venezuela]. Revista Ecológica Latino Americana. 2001;8:1–13.
- Jimenez JE, Jaksic JM. Behavioral ecology of Grey Eagle-Buzzards, Geranoaetus melanoleucus in Central Chile. Condor. 1989;91:913–921.10.2307/1368076
- Trujillo F, Trujillo J. Alimentación del lobo (Lycalopex culpaeus), en el bosque protector Jerusalem, Guayllabamba-Ecuador. Politécnica. 2007;27:68–75.
- Hernandez-Guzman A, Payan E, Monroy-Vilchis O. Food habits of Puma concolor (Carnivora: Felidae) in the Parque Nacional Natural Purace, Colombia. Revista de Biología Tropical. 2011;59:1285–1294.
- Bianchi RDC, Mendes SL. Ocelot (Leopardus pardalis) predation on primates in Caratinga Biological Station, Southeast Brazil. Am. J. Primatol. 2007;69:1173–1178.10.1002/(ISSN)1098-2345
- Kasper CB, Mazim FD, Soares JB, et al. Composition and relative abundance of the medium-large sized mammals of Turvo State Park, Rio Grande do Sul, Brazil. Revista Brasileira de Zoologia. 2007;24:1087–1100.10.1590/S0101-81752007000400028
- Cáceres NC, Nápoli RP, Casella J, et al. Mammals in a fragmented savannah landscape in south-western Brazil. J. Nat. History. 2010;44:491–512.10.1080/00222930903477768
- Bianchi RDC, Rosa AF, Gatti A, et al. Diet of margay, Leopardus wiedii, and jaguarundi, Puma yagouaroundi,(Carnivora: Felidae) in Atlantic Rainforest, Brazil. Zoologia (Curitiba). 2011;28:127–132.10.1590/S1984-46702011000100018
- Borges LH, Calouro A, Botelho AL, et al. Diversity and habitat preference of medium and large-sized mammals in an urban forest fragment of southwestern Amazon. Iheringia. Série Zoologia. 2014;104:168–174.10.1590/1678-476620141042168174
- Durant P. Ecological study of the hare Sylvilagus brasiliensis meridensis Lagomorpha Leporidae in the plateau of the Venezuelan Andes Caribbean. J. Sci. 1983;19:21–30.
- Ramsay PM, Oxley ERB. The growth form composition of plant communities in the Ecuadorian páramos. Plant Ecol. 1997;131:173–192.10.1023/A:1009796224479
- Novaro AJ, Capurro AF, Travaini A, et al. Pellet-count sampling based on spatial distribution: a case study of the European hare in Patagonia. Ecol. Austral. 1992;2:11–18.
- Murray DL, Roth JD, Ellsworth E, et al. Estimating low-density snowshoe hare populations using fecal pellet counts. Can. J. Zool. 2002;80:771–781.10.1139/z02-027
- Palomares F. Comparison of 3 methods to estimate rabbit abundance in a Mediterranean environment. Wildl. Soc. Bull. 2001;29:578–585.
- Forys EA, Humphrey SR. Comparison of 2 methods to estimate density of an endangered lagomorph. J. Wildl. Manage. 1997;61:86–92.10.2307/3802417
- Green JS, Flinders JT. Habitat and dietary relationships of the pygmy rabbit. J. Range Manage. 1980;33:136–142.10.2307/3898429
- Martínez-García JA, Mendoza GD, Sánchez-Trocino M, et al. Defecation rate in Romerolagus diazi fed with different levels of Muhlenbergia macroura. J. Appl. Anim. Res. 2011;39:317–319.10.1080/09712119.2011.607906
- Simonetti JA. Tasas de defecación y descomposición de fecas de Oryctolagus cuniculus en Chile Central [Defecation rates and feces decomposition of Oryctolagus cuniculus in central Chile]. Medio Ambiente. 1989;10:92–95.
- Siracusa AM, Petralia E. Trend of a population of Wild Rabbit Oryctolagus cuniculus (Linnaeus, 1758) in relation to Domestic Sheep Ovis aries aries (Linnaeus, 1758) grazing within a small insular protected area. Biodivers. J. 2013;4:557–564.
- Fernandez-de-Simon J, Díaz-Ruiz F, Cirilli F, et al. Towards a standardized index of European rabbit abundance in Iberian Mediterranean habitats. Eur. J. Wildl. Res. 2011;57:1091–1100.10.1007/s10344-011-0524-z
- Martínez-García, JA. Densidad, uso y evaluación del hábitat y de la dieta del Romerolagus diazi en el Parque Nacional Izta-Popo, Zoquiapan y anexas [Density, habitat use and evaluation, and diet of Romerolagus diazi in the National Izta-Popo Park, Zoquiapan and annex]. Doctorate Cience Dissertation. Institución de Enseñanza e Investigación en Ciencias Agrícolas, Mexico; 2011.
- Trout RC, Langton S, Smith GC, et al. Factors affecting the abundance of rabbits (Oryctolagus cuniculus) in England and Wales. J. Zool. 2000;252:227–238.10.1111/jzo.2000.252.issue-2
- Keating PL. Chronically disturbed paramo vegetation at a site in Southern Ecuador. J. Torrey Bot. Soc. 2000;162–171.10.2307/3088693
- Suárez E, Medina G. Vegetation structure and soil properties in Ecuadorian Páramo grasslands with different histories of burning and grazing. Arct. Antarct. Alp. Res. 2001;33:158–164.
- Ríos OV. Disturbios en los páramos andinos. Visión socioecosistémica de los páramos y la alta montaña colombiana: memorias del proceso de definición de criterios para la delimitación de páramos [Disturbances in the Andean páramos. Social economic view of Colombian páramos and high mountains: memories of the process of defining criteria for the delimitation of páramos]. Bogota: Instituto de Investigación de Recursos Biológicos Alexander von Humboldt; 2013; p. 39–57.