Abstract
The phenology, floral biology and pollination ecology of Salvia arborescens Urb. & Ekman (Lamiaceae) are reported, based on field and garden observations. The flowers of S. arborescens are white, fragrant and rich in nectar. Anthesis begins in the late afternoon, and flowers wilt by late morning of the following day. Floral features fit the moth pollination syndrome. S. arborescens flowers are visited by a variety of nocturnal moths in the wild, including Celiptera levinum (Lepidoptera: Noctuoidea: Erebidae), which was observed carrying pollen of this Salvia. This is the first report of a night-blooming, moth-pollinated Salvia. Hummingbirds, butterflies and beetles may play a role as secondary pollinators.
Introduction
The genus Salvia L., the sages, comprises ca. 1000 species in the tropics and temperate zones around the world. It is well-known for the unusual morphology of its stamens (the stamen ‘lever’) and for attracting a variety of birds, bees and flies to its flowers.[Citation1–4] Nevertheless, field studies of floral biology and pollination are strikingly few.[Citation5] Wester and Claßen-Bockhoff [Citation3] recently reviewed the literature on pollination of New World sages and assigned nearly all American species of Salvia to pollination syndromes. Salvia arborescens Urb. & Ekman (subgenus Calosphace section Wrightiana) was one of only eight species not classified, due to an absence of data.[Citation3]
S. arborescens has been found in the Cordillera Central (Dominican Republic), Massif de la Selle, de la Hotte and the Montagnes Noires (Haiti), on soils over limestone, along seepage slopes, water courses, streams and rivers at altitudes of 1150–1650 m.[Citation6] It was originally collected by Erik L. Ekman in Haiti in 1925 and named by Ignatz Urban and Ekman in 1926, after which it disappeared from notice. It was recollected in Haiti by Walter S. Judd in the late 1980s and a single population was discovered in the Dominican Republic by Alberto Veloz in the late 1990s; although in both cases, the collectors were uncertain about the identity of the Salvia and, consequently, unaware of the significance of their discoveries. Their specimens were confirmed to be S. arborescens during the course of a taxonomic revision of section Wrightiana.[Citation6] The species is now considered critically endangered within the Dominican Republic, with an area of occupancy less than 10 km2 and a population size estimated to number fewer than 50 mature individuals.[Citation7] Its conservation status in Haiti is unknown.
The senior author visited the only known extant population of S. arborescens and documented nocturnal anthesis and flower visitors in the wild, while the junior author collected floral biology data on plants in cultivation. Together, we undertook a study of its pollination biology. As most American species of Salvia are pollinated by either bees or hummingbirds,[Citation3] we expected bees or hummingbirds to be the main pollinators.
Methods
Flower morphology, flower color and flower life history, as well as nectar characteristics were documented in the field (by MR) and from cultivated plants in Miami, Florida, USA (by SZ). The field site was located at the known population of S. arborescens in the Dominican Republic, near Constanza in the Parque Nacional Valle Nuevo, in the Cordillera Central.[Citation6] Field observations were made (by MR) on 6–14 November 2013 and again on 18 December 2014.
Calipers were used to measure flower tube length, length of upper and lower lips, functional stamen length (distance from the apex of the corolla tube to the point of attachment of pollen sacs), length of pollen sacs and length of the style. Flower and calyx colors were matched to the RHS Mini Color Chart. A series of hourly photos (09:00–18:00 h on 11 August 2013, 07:00–08:00 h on 12 August 2013) provided data on flower phenology and average number of flowers per inflorescence.
Nectar standing crop was measured in unbagged flowers in the field at four intervals: shortly after flowers began to open (14:00 h), at dusk (18:00 h), at midnight (24:00 h) and in the early morning (06:00 h). Some flower buds were bagged, and total nectar content was evaluated the following day at 07:00 h. Nectar volumes were determined using calibrated micropipettes and calipers. Small volume nectar samples were pooled to measure sugar content. On cultivated plants, two inflorescences were protected from crawling insects by a Tanglefoot® barrier (The Tanglefoot Company, Grand Rapids, Michigan, USA) applied around the bases of the inflorescences. Nectar from protected flowers was collected at 07:00 h, using a pipette fitted with 10 μl round gel-loading tips (0.58 mm outside diameter; Thermo Fisher Scientific, Grand Island, New York, USA). The nectar was transferred into pre-weighed 0.5 ml microcentrifuge tubes and weighed in a balance to determine the mass of each nectar sample. A known volume of nectar was weighed separately to calculate the volumetric mass density, with which the nectar volume was calculated for each sample. Sugar content was recorded as % sugar (wt/wt), and was determined with a handheld refractometer (Belligham & Stanley Ltd., Tunbridge Wells, UK). Glucose test papers (Ames Clinistix, Miles Inc., Elkhart, Indiana, USA) were used to test for the presence of glucose in nectar. Anthers were collected at 06:30 from flowers in cultivation. Pollen was counted with a hemocytometer (Bright-Line 1492, Hausser Scientific, Horsham, Pennsylvania, USA) in order to calculate the P:O ratio.[Citation8]
Flower visitor observations were made between 06:00 and 24:00 h in the field. Special attention was given to Hymenoptera and hummingbirds, as the majority of New World Salvia sp. is pollinated by either bees or hummingbirds.[Citation3] A systematic survey of flower visitors was made during daylight hours, to quantify the diversity of visitors and the temporal patterns of visitation to flowers of S. arborescens. The 11.5 h between 06:45 and 18:15 h were subdivided into 46 observation periods of 15 min each. A single observation plot of nine inflorescences was selected; every flower visitor present in the plot at the start of the 15 min period and every flower visitor arriving in the plot was counted.
The systematic survey was complemented by nonsystematic observations in the field during daylight hours and during the night between 18:15 and 24:00 h, and in the garden between 12:00 and 24:00 h on the day of anthesis and at 06:00–11:00 h on the following morning. Photos of flower visitors were taken whenever possible. Nocturnal observations were made in the moonlight, aided by flash photography (EXILIM EX-ZR1000, Casio, Tokyo, Japan) and a flashlight. Flower visitation and pollen removal were documented from photos taken in the field. Sequential photographs of flower visitors, visiting a single inflorescence, were compared thoroughly. Pollen deposition on the visitor’s body, not present when the visitor was first photographed on the inflorescence but present later in the same series of photographs, was interpreted as clear signs of pollen uptake. Pollen loads were estimated from sequential photographs of foraging insects, and pollen identity was ascribed based on pollen color and foraging history of the insect. Given the rarity and small population size of this plant, we refrained from methods having the potential to interfere with its reproduction.
Results
Morphology
Flowers of S. arborescens are crowded onto dense, terminal, unbranched inflorescences bearing 16–20 flowers per verticillaster, although usually up to 10 flowers per inflorescence are open at any one time. The flowers face outward from the upright axis of the inflorescence at an angle of ca. 90°. The corolla is ca. 14 mm long, which includes the corolla tube that is 7.3 ± 0.2 mm (n = 10) long and reflexed upper lip (6.4 ± 0.4 mm (n = 10)) and lower corolla lip (7.9 ± 0.2 mm (n = 10)). Calyx color is yellow green (44 RHS 145B), while the tips of the calyx lobes match Dark Violet (22 RHS 83B). The corolla is white (59 RHS 157B) and lacks visible nectar guides. The stamens are inserted at the apex of the corolla tube, and the elongate connectives are inflexed in buds. The anthers are exserted 19.0 ± 0.8 mm (n = 10) beyond the mouth of the tube at anthesis. The ‘lever’ mechanism is nonfunctional because the sterile arms of the connective are appressed to the short, narrow floral tube leaving no room for them to move.[Citation2] Each pollen sac is medifixed, versatile and 3.2 ± 0.2 mm long (n = 10). The pollen is creamy white (60 RHS 155D). The long style also extends 15–20 mm beyond the corolla tube. The distance of the stigma to the base of the flower tube is 30.8 ± 0.9 mm (n = 10). The pistil has four ovules, which mature and separate to form four mericarps (nutlets).[Citation6] Plants in cultivation in Florida, USA, are all clones from the same parent plant. No fruit set was observed in cultivated plants. Pollination by hand in the morning after anthesis did not result in fruit set. The mean estimated pollen/ovule ratio is 2980 ± 1295 (n = 5).
Phenology
The flowering season of plants in situ and in cultivation during 2013–2015 was November and December. Flowers opened slowly then senesced and dropped in a synchronized way. A flower cohort (the flowers opening on the same day) lasted about 28 h, with an overlap with the next cohort of only 4 h (Figure ).
Figure 1. Number of open flowers per inflorescence, showing the complete lifetime of one flower cohort (cohort 2) and overlap with the previous and the subsequent flower cohorts (cohorts 1 and 3, respectively).
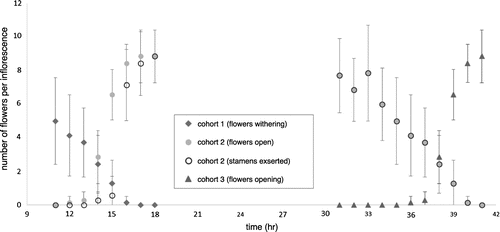
During anthesis, the position of stigma and anthers changes considerably, as styles and stamen connectives are contained in an inflexed position within the bud. As flowers first began to open (14:00 h), the styles emerged, followed by the stamen connectives (Figure ). Following emergence, styles and connectives began to straighten (Figure ). Shortly before sunset (local sunset was 17:36 h in Miami and 18:05 h in the Dominican Republic), the corolla opened, the connectives largely unfurled and the flowers began to emit a sweet fragrance. The anthers began dehiscing at this time. By 20:00 h, the flowers were fully open and fragrant. The fragrance was not strong (one had to be close to the flower to detect it), but it was sweet and pleasant. By 06:00 h of the following day, the flowers were still open (Figure ), but the fragrance was no longer detectable. Between 08:00 and 16:00 h of day two, the flowers (including the stamens) withered and fell from the inflorescence.
Nectar
Shortly after flowers opened (14:00 h), nectar volume in unbagged flowers in the wild was 0.05 ± 0.09 μl (n = 10), rising to 0.58 ± 0.53 μl (n = 10) at dusk (18:00hr) and 3.23 ± 2.81 μl (n = 10) by midnight. By early morning of the following day (06:00 h), the nectar crop was only 0.59 ± 0.4 μl (n = 10). Bagged flowers in the wild were measured to contain 21.25 ± 4.57 μl (n = 10) at 07:00 h on the morning following anthesis (but some nectar may have been lost as the flowers were inadvertently jostled during bag removal). In cultivated plants with Tanglefoot® barriers, nectar accumulated in the corolla tube until it became visible as a bulging droplet at the mouth of the tube. Nectar volume at 07:00 h was 81.27 ± 6.48 μl (n = 10) in unvisited flowers.
Sugar content in wild plants began at 16.8 ± 0.5% (n = 5) at 18:00 h, declining to 14.8 ± 1.1% (n = 10) at midnight, and falling to 12.2 ± 2.9% (n = 6) by 06:00 h of the following morning. Sugar content in unvisited (bagged) flowers was 10.0 ± 1.6% (n = 10) at 07:00 h on the morning after anthesis. In cultivated plants, the sugar concentration of nectar at 07:00 h was 17.3 ± 1.6% (n = 10). Glucose test papers revealed no glucose present in the nectar.
Flower visitors
In the wild, members of four insect orders (Hymenoptera, Diptera, Lepidoptera and Coleoptera) (Figure ) and hummingbirds visited the flowers of S. arborescens. The systematic survey during daylight hours found that the non-native Western honeybee (Apis mellifera) was the most abundant flower visitor within this timeframe (244 recordings: 237 collecting nectar, 7 collecting pollen). Most honeybees were seen collecting nectar from newly opened flowers in the afternoon or from old flowers on the morning following anthesis (Figure ). A separate cohort of honeybees was also observed around noon collecting pollen from withering flowers (Figure ). Nectar-collecting honeybees did not carry pollen, and flower morphology of S. arborescens prevented them from touching anthers or stigma during their visits. Pollen-collecting honeybees carried loads of pollen on their hind legs and were observed collecting more pollen from S. arborescens anthers.
Figure 4. Numbers of insects visiting Salvia arborescens flowers during systematic observation periods (afternoon of the first day of flowering; morning of the second day).
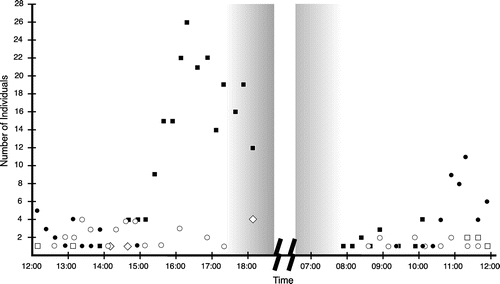
Most of the Diptera recorded during the systematic survey (44 recordings) were small hoverflies (Syrphidae). No clear peak of activity was observed. Most syrphid flies landed directly on the anthers and consumed pollen. Syrphid flies were not seen carrying the easily visible pollen of S. arborescens.
Several species of Lepidoptera were observed during the systematic survey (74 recordings). The most abundant species being the endemic Hispaniolan King butterfly, Anetia jaegeri (Ménétriés) (Nymphalidae: Danainae), with 66 recordings. Activity of the Hispaniolan King peaked on old, withering flowers before noon (Figure ). Individuals of this Hispaniolan endemic butterfly were seen transporting on their wings and antennae pollen that matched in color the pollen of S. arborescens. Field observations on pollen uptake and pollen transport by A. jaegeri were also documented by photos. Several series of photographs of single butterflies visiting single inflorescences show pollen deposits on the butterflies that increase during the visits, which indicates that A. jaegeri took up and carried pollen of S. arborescens. Another diurnal visitor to S. arborescens was the Violet-banded skipper, Nyctelius nyctelius (Latreille) (Hesperiidae: Hesperiinae), for which there were two recordings. We obtained no conclusive evidence for pollen uptake or pollen transport by this rare visitor.
Diurnal visits of moths were also rare (six recordings), and no pollen uptake and pollen transport was documented in their diurnal visits. Moth activity increased at dusk (four recordings), just prior to the end of the systematic observation period (Figure ). More moths were seen in the non-systematic survey, during dawn and at night in the moonlight, hovering at or near the inflorescences of S. arborescens. Some moths landed on inflorescences and were directly observed probing the flowers for nectar. Different kinds of moths were spotted in the light of the flashlight, and the reflective eyes of moths were observed in the red light of the optical distance sensor of the camera; however, most moths fled artificial light sources and could not be identified further. We were able to make a series of photographs of a settling moth, Celiptera levinum (Stoll) (Noctuoidea: Erebidae), visiting an inflorescence of S. arborescens. The position and color of the pollen on the first photo of the series strongly suggest that it came from S. arborescens. In addition, the area covered with pollen on the moth’s left forewing (Figure ) increases with time in this series of photos. This documents that this moth was taking up and transporting pollen of S. arborescens. An unidentified species of ground beetle (Coleoptera: Carabidae) was another nocturnal flower visitor, pollen was also observed on this beetle.
Figure 5. Celiptera levinum (Lepidoptera: Noctuoidea: Erebidae) visiting Salvia arborescens in the Dominican Republic. Source: Photo by M. Reith.

While hummingbirds were not observed visiting flowers during the systematic survey, 11 visits of hummingbirds at S. arborescens flowers were noted during 10 days of non-systematic observations. Two species were observed: the Antillean Mango (Anthracothorax dominicus) and Hispaniolan Emerald (Chlorostilbon swainsonii). Visits by hummingbirds to the flowers of S. arborescens were very rare, and they often occurred at dusk. We obtained no evidence for pollen transport by hummingbirds.
The most frequent nocturnal visitor to flowers cultivated in Florida was the carpenter ant (Camponotus floridanus), which collected nectar. An unidentified coleopteran (Scarabaeidae; possibly Euphoria sepulcralis) was observed one time feeding on nectar at night. By early morning, the flowers were visited by Western honeybees (Apis mellifera), which readily drained the flowers of remaining nectar. All of these insects entered the flower at its base, either by landing on the edge of the corolla (bees) or climbing up from the outside of the calyx and corolla (ants, beetle). In doing so, none of these insects came in contact with the anthers or stigma; all were nectar thieves. A single Ruby-throated hummingbird (Archilochus colubris) was twice observed visiting the flowers at 07:00 h, but its function as a pollen vector could not be ascertained.
Discussion
This is the first report of a Salvia species that flowers at night. Many species of Salvia have flowers that last several days and are thus open during the night; others (e.g. Salvia costaricensis Oerst.[Citation9]) open in the darkness of predawn. S. arborescens is the only known species with flowers that begin to open in the late afternoon, reach full anthesis after sunset, and senesce and fall in following morning.
Our phenological observations suggest that pollination and fertilization occur at dusk, at the earliest, or later in the night. By morning of the following day, the flowers lose their fragrance and begin to senesce. Pollination at this late stage may not lead to fertilization, given the length that the pollen tubes must grow before the flower falls. Miyajima,[Citation10] working on Salvia splendens Sellow ex Schult., another species with a long style, showed that pollen requires 3 h to fertilize ovules, and pollen deposited on the stigma in the last third of a flower’s lifetime contributes significantly less to seed set than pollen deposited earlier. If this finding applies to other long-styled Salvia, such as S. arborescens, then pollinations in the morning do not significantly contribute to reproductive success. Our own experiments with hand pollination of S. arborescens in Florida point in this direction. While no self-incompatibility is known to occur in Salvia,[Citation11–13] hand pollination of flowers near the end of their lifetime, in the morning after anthesis, did not result in any fruit or seed set.
The floral visitors to S. arborescens include several potential pollinators. Pollen thieves (e.g. syrphid flies) and nectar thieves (e.g. honeybees) can be excluded from consideration, as they were not observed contacting both anthers and stigmas. Several other visitors (e.g. the Violet-banded skipper) were rare and unlikely to be significant pollinators. The remaining visitors can be assessed as to their ability to affect pollination based on their frequency, behavior, timing and observed pollen loads.
Diurnal flower visitors that were observed transporting pollen of S. arborescens in the wild are the Hispaniolan King butterfly, Anetia jaegeri, (most abundant) and pollen-collecting honeybees (rare). Butterfly visitors to Salvia flowers are usually regarded as poor pollinators or nectar thieves.[Citation9,14] The activity of the Hispaniolan King peaked between 11:00 and 12:00 h, when half of the flowers from the previous night have already dropped and one-half to two-thirds of the remaining flowers will drop within the next three hours. Hardly any newly opening flowers are available at this time. This means that any pollen transferred by the Hispaniolan King during its peak activity was among old flowers that were about to drop. Pollen-collecting honeybees were observed between 11:15 and 13:15 h, and all of the above arguments against pollination by the Hispaniolan King also apply to pollen-collecting honeybees.
The situation is slightly different for very early morning visits of the Hispaniolan King, or for the rare late visit of this species. Very early morning Hispaniolan Kings might encounter flowers that are few hours younger; late visiting Hispaniolan Kings might visit a newly opened flower and transfer pollen collected previously from an old flower. Such visits may contribute to seed set. Both early morning and late visits of the Hispaniolan King are, however, very rare. The large number of honeybees searching for nectar on freshly opened flowers in the afternoon might exclude or out-compete other visitors. Prior to the introduction of the honeybee, butterflies like the Hispaniolan King may have commonly visited the flowers of S. arborescens in the late afternoon, and they may have been more likely to affect pollination than they are now. We therefore conclude that visits by the Hispaniolan King butterfly, though abundant at 11:00–12:00 h, are of minor importance for the reproduction of S. arborescens. Visits by pollen-collecting honeybees are even less important, as they were rare to begin with, observed only on old flowers and were restricted to a timeframe in which pollination is least effective.
Several very rare diurnal flower visitors, like the Violet-banded skipper, diurnal moths and hummingbirds were not observed transporting pollen of S. arborescens, but they have the potential to do so. Due to their rarity, they are unlikely to be of major importance for the reproduction of S. arborescens. It is worth noting, however, that the peak of activity for diurnal moths and hummingbirds was close to dawn, so they did not visit young, freshly opened flowers. This makes them potential pollen vectors of minor importance for S. arborescens. As mentioned above, the introduction of the non-native Western honeybee may have changed the pollination dynamics for S. arborescens. The present-day abundance of nectar-foraging honeybees may alter the behavior of native hummingbirds; if their dusk visits were more abundant, they might play a greater role in the pollination of S. arborescens.
Nocturnal flower visitors, on the other hand, are active within a timeframe in which pollination is likely to be most effective, within the first two-thirds of a flower’s lifetime. At night, the flowers are fully open, and the stigmas and stamens occupy a similar position. Nocturnal flower visitors therefore have, if they contact anthers and take up pollen, a much greater chance of depositing pollen on receptive stigmas. All nocturnal flower visitors that we observed in detail in the wild were shown to transport pollen. These include the settling moth Celiptera levinum and an unidentified ground beetle. The taxonomic range of nocturnal moths visiting S. arborescens could not be fully explored due to the fact that most moths could not be identified in the field, as they fled every light source. While both nocturnal moths and beetles might be pollinators, we assume that moths are qualitatively better pollinators, as they were actively flying from inflorescence to inflorescence, in contrast to the ground beetle that was sedentary.
Observations on cultivated plants largely confirmed observations in the wild. Nectar collecting honeybees, while being the most abundant diurnal flower visitors, did not contact anthers and stigmas and did not transport pollen. Visitation of hummingbirds was very rare in cultivation. No moths were observed visiting cultivated plants, but observations of flower-visiting moths are infrequent generally.[Citation15–19]
The moth-pollination syndrome (phalaenophily), as summarized by Rosas-Guerrero et al.,[Citation20] includes the following features: anthesis nocturnal, color white, odor sweet and moderate to strong intensity, flower bell-, brush- or tube-shaped, symmetry radial, size medium-to-very large (corolla length 15 mm or more in length), orientation upright, horizontal or pendant, nectar guides absent, sexual organs enclosed, and nectar reward present. The flowers of S. arborescens meet all of these criteria, with the exception of odor intensity (weak in S. arborescens), corolla symmetry (zygomorphic), corolla length (small) and sexual organs enclosed (strongly exserted). Nevertheless, the suite of characters from S. arborescens comes closer to phalaenophily than to any other syndrome outlined in Rosas-Guerrero et al.[Citation20]
Nectar volumes of flowers exposed to nocturnal visitors in the wild were much lower than the nectar volumes of unvisited flowers. This finding indicates that nocturnal flower visitors consumed ample amounts of nectar. The mean nectar concentration (17.3%) is in line with values reported for hawkmoth-pollinated plants, ca. 15–30%.[Citation21–25] Of the three sugars most commonly found in nectars, two are hexose monosaccharides (glucose and fructose) and one (sucrose) is a disaccharide. Baker and Baker [Citation21] summarized their extensive research in nectar sugars and found the following: glucose occasionally is the sole sugar in nectar but more commonly co-occurs with fructose and/or sucrose; sucrose may occur as the sole sugar source but never co-occurs with only fructose. Fructose never occurs as the sole sugar. Our partial analysis of nectar sugars indicated an absence of glucose and, by implication, an absence of its frequent partner fructose. We thus infer that the primary nectar sugar in S. arborescens is sucrose. This conclusion is in line with the observation that the Lamiaceae typically produce sucrose-rich nectars.[Citation24,25] Among nocturnally pollinated plants, sucrose-rich or sucrose-dominant nectars are typical of moth-pollinated plants, in contrast to the hexose-rich nectars of species pollinated by nocturnal, non-volant mammals and Neotropical bats.[Citation21]
Floral fragrance is rare in Salvia.[Citation3] The fragrance of S. arborescens is not strong, so it is unlike that of many familiar moth-pollinated flowers (e.g. species of Nicotiana, Brunfelsia, Cubanola). The scent of S. arborescens is not detectable by morning. Scent production at dusk and into the night suggests that it plays an important role in attracting nocturnal floral visitors.
The P:O ratio of S. arborescens (2980 ± 1295) is higher than mean P:O ratios for bee-pollinated species of Salvia (mean P:O = 1400.8; n = 12), higher than that of perching bird-pollinated species (mean P:O = 740.7; n = 2) but lower than that of hummingbird-pollinated species (mean P:O = 6113.2; n = 6) (Supplement 1). High P:O ratio may be related to a large pollen-bearing (source) area and a small stigmatic (target) area.[Citation26] Exserted stamens deposit pollen on the head, body and wings of moths [Citation15,19]; thus pollen-bearing moths may present a large source area relative to the target. The stigma of S. arborescens is small – an acute terminus of a slender style. The lower probability of pollen reaching the stigma may explain, in part, the high P:O ratio.[Citation8] The P:O ratio of S. arborescens is in the range of predominately outcrossing taxa.[Citation8,27]
The flower shape, position, color, sweet fragrance, nectar reward, composition and crepuscular/nocturnal anthesis suggest pollination by moths (phalaenophily). Field observations confirmed nocturnal visits by moths and that nocturnal moths were transporting pollen of S. arborescens. While pollen deposition by moths was not shown, the evidence strongly suggests that moths play an important role as pollinators of S. arborescens. This is the first and only putative case of moth pollination in the genus Salvia, the only case of moth pollination of Lamiaceae in the Neotropics, and only the third case globally.[Citation28] Nocturnal beetles, dusk visits by hummingbirds and moths, or early morning and late afternoon visits by butterflies may contribute to pollination assurance.
Further study of S. arborescens in its natural habitat would clarify the relative importance of the beetles, hummingbirds, butterflies and moths in affecting pollination. We did not directly sample pollen loads from these organisms, nor did we make visitor observations throughout the night. Other laboratory observations, such as additional P:O ratio counts, examination of stigmas for wing scales of moths, and analyses of fragrance chemistry, would also be helpful in understanding the roles of animals, especially moths, in the pollination of this species of Salvia.
Acknowledgments
We thank Kate Wallace for generously introducing the senior author to the hummingbirds of Hispaniola, and Teodoro Clase and Alberto Veloz, without whom this intriguing Salvia would have remained unexamined. Dr. Suzanne Koptur, in whose lab much of the analytical work was done, generously provided guidance on the project and comments on the manuscript. Two anonymous reviewers provided helpful comments on earlier drafts of the manuscript. This is contribution 322 to FIU’s Tropical Biology series.
Associate Editor: Nora Oleas.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/23766808.2016.1230461.
Supplement_1_published_p-o_ratios_neotrop_biodiv_style_1_.docx
Download MS Word (13.1 KB)References
- Wester P, Claßen-Bockhoff R. Bird pollination in South African Salvia species. Flora. 2006a;201:396–406.10.1016/j.flora.2005.07.016
- Wester P, Claßen-Bockhoff R. Hummingbird pollination in Salvia haenkei (Lamiaceae) lacking the typical lever mechanism. Plant Syst. Evol. 2006b;257:133–146.10.1007/s00606-005-0366-9
- Wester P, Claßen-Bockhoff R. Pollination syndromes of New World Salvia species with special reference to bird pollination. Ann. Mo. Bot. Gard. 2011;98:101–155.10.3417/2007035
- Celep F, Atalay Z, Dikmen F, et al. Flies as pollinators of melittophilous Salvia species (Lamiaceae). Am. J. Bot. 2014;101:2148–2159.10.3732/ajb.1400422
- Claßen-Bockhoff R, Wester P, Tweraser E. The staminal lever mechanism in Salvia L. (Lamiaceae) – a review. Plant Biol. 2003;5:33–41.10.1055/s-2003-37973
- Zona S, Clase T, Franck A. A synopsis of Salvia section Wrightiana (Lamiaceae). Harvard Pap. Bot. 2011;16:383–388.10.3100/0.25.016.0208
- Ministerio de Medio Ambiente y Recursos Naturales de la República Dominicana. Lista de Especies in Peligro de Extinción, Amenazadas o Protegidas de la República Dominicana (lista roja) [List of Endangered, Threatened and Protected Species in the Dominican Republic (red list)]. Santo Domingo: Ministerio de Medio Ambiente y Recursos Naturales de la República Dominicana; 2011.
- Cruden RW. Pollen-ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution. 1977;31:32–46.10.2307/2407542
- Hedström I. Nattaktiva bin som pollinatörer av en tropisk Salvia [Nocturnal bees as pollinators of a tropical Salvia] Fauna Flora. 1985;80:101–110.
- Miyajima D. Seed-producing system in Salvias. J. Am. Soc. Hortic. Sci. 1996;3:419–422.
- Haque MS, Goshal KK. Floral biology and breeding system in the genus Salvia L. Proc. Indian Nat. Sci. 1981;5:716–724.
- Navarro L. Is the dichogamy of Salvia verbenaca (Lamiaceae) an effective barrier to self-fertilization? Plant Syst. Evol. 1997;207:111–117.10.1007/BF00985212
- Zhang B, Claßen-Bockhoff R, Zhang Z-Q, et al. Functional implications of the staminal lever mechanism in Salvia cyclostegia (Lamiaceae). Ann. Bot. 2011;107:621–628.10.1093/aob/mcr011
- Espino Espino J, Baños Bravo YE, Cuevas García E. Biología reproductiva y visitantes florales de dos especies de Salvia con síndrome de polinización por aves y abejas. Cienc. Nicolaita. 2012;55:52–60.
- Baker HG. The adaptation of flowering plants to nocturnal and crepuscular pollinators. Q. Rev. Biol. 1961;36:64–73.
- Wolff D, Braun M, Liede S. Nocturnal versus diurnal pollination success in Isertia laevis (Rubiaceae): a sphingophilous plant visited by hummingbirds. Plant Biol. 2003;5:71–78.10.1055/s-2003-37977
- Oliveira PE, Gibbs PE, Barbosa AA. Moth pollination of woody species in the cerrados of Central Brazil: a case of so much owed to so few? Plant Syst. Evol. 2004;245:41–54.
- Fox K, Vitt P, Anderson K, et al. Pollination of a threatened orchid by an introduced hawk moth species in the tallgrass prairie of North America. Biol. Conserv. 2013;167:316–324.10.1016/j.biocon.2013.08.026
- Atwater MM. Diversity and nectar hosts of flower-settling moths within a Florida sandhill ecosystem. J. Nat. Hist. 2013;47:2719–2734.10.1080/00222933.2013.791944
- Rosas-Guerrero V, Aguilar R, Martén-Rodríguez S, et al. A quantitative review of pollination syndromes: do floral traits predict effective pollinators? Ecol. Lett. 2014;17:388–400.10.1111/ele.2014.17.issue-3
- Baker HG, Baker I. Chemical constituents of nectar in relation to pollination mechanisms and phylogeny. In: Nitecki HM, editor. Biochemical aspects of evolutionary biology. Chicago, IL: University of Chicago Press; 1982. p. 131–171.
- Perret M, Chautems A, Spichiger R, et al. Nectar sugar composition in relation to pollination syndromes in Sinningieae (Gesneriaceae). Ann. Bot. 2001;87:267–273.10.1006/anbo.2000.1331
- Kaczorowski RL, Gardener MC, Holtsford TP. Nectar traits in Nicotiana section Alatae (Solanaceae) in relation to floral traits, pollinators, and mating system. Am. J. Bot. 2005;92:1270–1283.10.3732/ajb.92.8.1270
- Percival MS. Types of nectar in angiosperms. New Phytol. 1961;60:235–281.10.1111/nph.1961.60.issue-3
- Stiles FG, Freeman CE. Patterns in floral nectar characteristics of some bird-visited plant species from Costa Rica. Biotropica. 1993;25:191–205.10.2307/2389183
- Cruden RW. Pollen grains: why so many? Plant Syst. Evol. 2000;222:143–165.10.1007/BF00984100
- Michalski SG, Durka W. Pollination mode and life form strongly affect the relation between mating system and pollen to ovule ratios. New Phytol. 2009;183:470–479.10.1111/j.1469-8137.2009.02861.x
- Macgregor CJ, Pocock MJO, Fox R, et al. Pollination by nocturnal Lepidoptera, and the effects of light pollution: a review. Ecol. Entomol. 2015;40:187–198.10.1111/een.12174


