Abstract
Resin bugs are a group of reduviids which collect viscous substances from plants with their legs and use them as an aid in prey capture and to protect eggs against predators. We designed the present investigation to examine aspects of the natural history and biology of Heniartes stali (Wygodzinsky) in association with Rubus cf. adenotrichos (Schltdl.), an Andean wild blackberry covered with trichomes that secrete a sticky fluid. We surveyed the occupancy of this plant by the bugs in five localities in the Venezuelan Andes and the sticky material source was determined. Nymphs, and in minor proportion, adults were frequently found on the blackberry plants. We also document the harvesting and handling of this exudate by H. stali under laboratory conditions. The bugs invested several hours to gather the sticky material using the forelegs, transferred it to the midlegs, and subsequently stored it in a conspicuously hairy area of the hindlegs. Portions of the accumulated material were smeared on the genital area using the hind tibiae for coating the eggs during oviposition. We also observed the transfer from the hind to the forelegs. Our observations reveal a previously unsuspected interaction between Heniartes and trichome-bearing plants in the Neotropics. In addition, we report hitherto unknown substantial differences in the way resinous fluids are handled by Heniartes, in contrast with reports of other Apiomerini.
Los chinches de las resinas son un grupo de redúvidos que recogen sustancias viscosas de las plantas con sus patas y las utilizan para ayudarse en la captura de las presas y para proteger los huevos de los depredadores. La presente investigación fue diseñada para examinar aspectos de la historia natural y biología de Heniartes stali (Wygodzinsky) en asociación con Rubus cf. adenotrichos (Schltdl.), una mora andina cubierta de tricomas que secreta un fluido pegajoso. La ocupación de esta planta por los chinches fue examinada en cinco lugares de los Andes de Venezuela y se determinó el origen del material pegajoso. Las ninfas, y en menor proporción, los adultos se encontraron con frecuencia en las plantas de mora. También se describe la recolección y manipulación de este exudado por H. stali en condiciones de laboratorio. Los chinches invirtieron varias horas para recolectar el material pegajoso usando las patas delanteras, transferirlo a las patas medias y posteriormente almacenarlo en una zona visiblemente pilosa de las patas posteriores. Con éstas las hembras untaban el área genital con la secreción para el recubrimiento de los huevos durante la oviposición. También se observó la transferencia hacia las patas anteriores desde el reservorio en las patas posteriores. Nuestros resultados demuestran la interacción inédita de Heniartes con una planta que posee tricomas glandulares en el Neotrópico. Además, reportamos diferencias importantes en relación con la manipulación de la secreción pegajosa en comparación con lo conocido para otros Apiomerini.
Introduction
The new world tribe Apiomerini is remarkable among Reduviidae because of some of its species, known as resin bugs, are adapted to collect viscous substances.[Citation1,2] The sticky properties of the resin assist in prey capture and protect the eggs against predators, parasitism or desiccation.[Citation3] Only few of the published studies describe the plant resin sources, the resin-collecting morphological structures and behavior of the bugs during resin harvesting. The best studied species to explain the origin, gathering and function of the resin is the Nearctic Apiomerus flaviventris.[Citation3–5] Females gathered droplets of resin from the camphor weed Heterotheca psammophila (B. Wagenkn.) (Asteraceae) using the forelegs, then transferred them to the midlegs and from there to the ventral surface of the abdomen by rubbing their hindlegs. The material thus collected was used afterwards during oviposition to cover eggs with a resin layer which allegedly protected them against predation or microbial attack.
In the Neotropics, early reports on Heniartes jaakkoi (Wygodzinsky) emphasized the presence of a ‘viscous liquid,’ chiefly on legs.[Citation6] This author suggested the possible use of this material as a viscous trap to capture prey but did not determine its source.
More recently, a gummy substance on the forelegs of early instars of H. jaakkoi was recorded.[Citation7] Insects were reared in the laboratory without access to any resinous source, which suggests it to be a secretion from the insect or material collected from eggs and/or exuviae. The actual origin was not established, though.
Our early field observations evidenced the occurrence of Heniartes stali on the Andean blackberry R. cf. adenotrichos and the recurrence of a sticky material on their legs and eggs laid in the laboratory. H. stali was described [Citation8] based on specimens from Mérida, Venezuela, although geographical distribution, biology and behavior were not reported. Rubus cf. adenotrichos (Schltdl.) (Rosaceae) belongs to a group of tropical highland blackberries of complex taxonomy as a result of prevalent hybridization, polyploidy, and agamospermy.[Citation9,10] Neotropical Rubus are distributed from Mexico to Ecuador in wild thickets or cultivated for their edible polyphenolics-rich fruits.[Citation11] One of the most conspicuous features of R. cf. adenotrichos is the abundant coverage of glandular trichomes on stems and budding structures which secrete a sticky exudate. To the best of our knowledge, the chemical composition and possible biological and ecological roles of this exudate remain unreported. It is nonetheless known that the viscous secretions from trichome-bearing plants generally serve as a defense barrier against phytophagous arthropods and other organisms.[Citation12,13]
It thus seemed an excellent opportunity to study aspects of the natural history and biology of the possible interaction of a neotropical resin bug with plant species endowed with a gummy trichome secretion on its stems. We hypothesized that H. stali might collect the trichome secretion (hereafter TS) of R. cf. adenotrichos.
To this end, we combined field records and laboratory observations of H. stali nymphs and adults on R. cf. adenotrichos to address the following questions: (1) Is R. cf. adenotrichos frequently occupied by H. stali? (2) Is R. cf. adenotrichos a TS source to H. stali? (3) How does H. stali gather and manipulate the TS?
Material and methods
Occupation of R. cf. adenotrichos by H. stali
We conducted a survey to study the temporal occupancy of R. cf. adenotrichos by H. stali in El Tanque, an area of Cerro La Bandera, near Mérida city (8°31′N-71°13′W, 1980 m), in the Andean range of Venezuela. The vegetation in this site consists of fragmented secondary forests with arboreal elements, shrubs, ferns, and grasses. Some of the most abundant plant species in the area are bracken fern Pteridium spp. (Dennstaedtiaceae), Chamaecrista sp. Acacia sp. (Fabaceae), Clusia sp. (Clusiaceae), Rubus spp. (Rosaceae), Psidium sp.(Myrtaceae), Monochaetum sp. (Melastomataceae), and the Poaceae Pennisetum purpureum and Melinis minutiflora.[Citation14] Rainfall and average annual temperature is 2000 mm and 16 °C, respectively, with a bimodal April–July and September–November rain regime.[Citation14]
We visited El Tanque between 8.00 and 11.00 am twice a month, from January 2013–January 2014. We conducted four additional surveys during January and February 2015. In each visit, we randomly inspected 12–14 blackberry plants from a total amount of 25 individuals that were monitored at the start of the study, growing sparsely at each side along a 1050-m-long trail. We examined all leaves and stems of the blackberry plants below 2.5 m height for 5–10 min. We recorded the presence of eggs, nymphs, adults, and exuviae of H. stali. The field identification of the development stages of the bug was facilitated by previous observations of the life cycle in the laboratory. We collected the adults to determine the sex in the field by visual examination of the external genitalia [Citation8] and subsequently released.
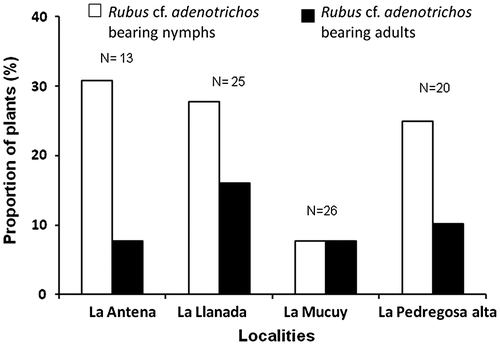
Also, we assessed the occupancy of blackberry plants by H. stali through one-day surveys in four additional localities during March–April 2015: La Antena (8°31′N-71°13′W, 2080 m), La Llanada (8°36′N-71°10′W, 2150 m), La Pedregosa Alta (8°36′N-71°11′W, 2000 m), and La Mucuy (Sierra Nevada National Park, 8°37′N-71°02′W, 2200 m). All sites have similar vegetation and climate of Cerro La Bandera. In each locality, we inspected between 13 and 26 individuals of the blackberry plants as described above.
Voucher specimens of H. stali and R. cf. adenotrichos were deposited in the insect collection of the Laboratorio de Ecología de Insectos (CLEI-ULA) and the herbarium of the Facultad de Ciencias Ambientales y Forestales (MER), respectively, both institutions of Universidad de Los Andes, Mérida, Venezuela.
Study of the insect sticky material source
We collected H. stali fifth instar nymphs (N = 2), females (N = 2) and males (N = 2) showing visible leg sticky material accumulation on R.cf. adenotrichos in the field. Bug metatibiae were carefully flushed with 1 ml dichloromethane to remove the sticky material and collected in a 3 ml vial. Additionally, we performed the analysis to the egg sticky cover as follows: individual gravid females of H. stali (N = 3) were exposed to R.cf. adenotrichos stems in the laboratory. Once bugs exhibited a visible sticky material in its legs, they were individually moved to a glass Petri dish and allowed to oviposit on the glass surface. Then, eggs (N = 12) were pooled and washed briefly with 1 ml dichloromethane which was collected in a 3 ml vial. Extracts of TS were obtained by briefly immersing small stem sections (2 cm long) of R. cf. adenotrichos in 5 ml dichloromethane.
Aliquots of all extracts were transferred to 2 ml vials and the solvent blown-dry under a current of air. Residues were redissolved in 500 μl dichloromethane and 5 μl samples were sowed on a thin layer chromatography plate (silica gel 60 F254 0.25 mm, Merck) and developed in a hexane-solvent mixture (2:1) to separate and compare possible common compounds between the spot patterns obtained from the samples. A mixture (1:1) of sulfuric acid and aqueous methanol (80%) was used to develop the spots.[Citation3]
Behavior of TS gathering and handling by H. stali
We performed behavioral studies in the laboratory using individuals obtained from a colony which was started from 27 eggs of 5 females collected in Cerro La Bandera site. The rearing conditions were as follows: Nymphs and adults were kept separately (one individual per plate) in glass Petri dishes (9 cm diameter) with filter paper lining. Nymphs were fed every second day with late instar larvae of Tribolium castaneum (Herbst) (Coleoptera:Tenebrionidae) from our laboratory stock colonies, whereas adults were fed with T. castaneum larvae and also with field collected workers of Camponotus rufipes (Fabricius) (Hymenoptera: Formicidae), which we frequently observed on the blackberry plants at the study sites. Moisture was supplied to each Petri dish with a small cotton swab soaked with aqueous honey (15% v/v). Rearing plates were kept in an acclimatized room under temperature of 22 ± 1 °C; 70 ± 10% RH and 12/12 h light/dark cycle.
We studied the behavior of H. stali to blackberry plant placing individual adult females (N = 12) in a glass Petri dish (9 cm diameter) with a freshly excised stem segment of R.cf. adenotrichos. The response of the bugs was monitored during 20-min periods once every hour for a total of 9 h, recording the strategy, anatomical parts involved, TS redistribution on the insect body and timing. Additional, we made unsystematic observations on juvenile stages (first instar (N = 16); second to fourth (N = 14); fifth (N = 12) and adult males (N = 5)) in order to detect possible variants of resin harvesting and handling behavior. We also performed contingent observations on the oviposition behavior of captive gravid females (N = 6) which had each been provided with a fresh segment of A. cf. adenotrichos showing trichome cover. The sequences were photographed, digitally videoed (Sony Cybershot® DSC-T90), and the number of individuals displaying each behavioral event were recorded. We named the insect anatomical parts involved after Forero et al. [Citation5].
Statistical analysis
The differences from a theoretical random distribution of the occurrence of nymphs and adults on R. cf. adenotrichos were tested using chi-square. Statistical analysis was performed using Statistix 9® (Analytical Software, Tallahassee, FL).
Ethics statement
This study was conducted according all relevant codes of experimentation and legislation.
Results
Occupation of R. cf. adenotrichos by H. stali
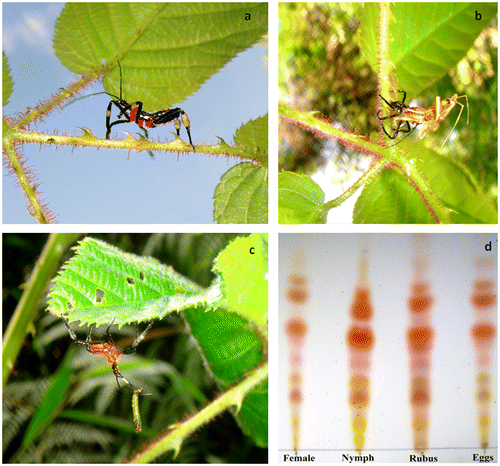
We observed nymphs on blackberry plants in every visit along the entire observation period at El Tanque (Figure ). The mean proportion of plants bearing nymphs was 12.53% ± 4.03% (±SD). We recorded a minor proportion of adult-bearing plants (1.33% ± 2.47%; X2 = 35.31; p < 0.01) and for a distinctively shorter period (4 out of 14 months). A total of 53 individual H. stali were observed on the surveyed plants of R. cf. adenotrichos during the field study period (48 nymphs, 3 adult females, and 2 adult males). Nymphs and adults were spotted well exposed on leaves but had a tendency to hide when lightly disturbed, or drop to the ground when substantially perturbed. Bugs were not gregarious, in most cases only one nymph per plant was observed, while up to three individuals were found in a single R.cf. adenotrichos plant on one occasion. Both nymphs and adults showed a coat of a viscous substance on their legs, chiefly on the metatibiae (Figure (a)). Exuviae were frequently found on petioles and stems (Figure (b)). The predatory habit of this species was suggested by our casual finding of a nymph with a captured caterpillar (Figure (c)), another one feeding on a worker of the ant Pheidole sp., and an adult male grabbing a chrysomelid beetle. We did not detect eggs on any plant parts throughout field observations. We frequently observed aphids and lepidopteran larvae feeding on the leaves. Also, we noted dead insects such as dipterans, small ants, and other unidentified hymenopterans trapped by the glandular trichomes.
Figure 1. Proportion of Rubus cf. adenotrichos individuals bearing Heniartes stali in El Tanque, Cerro La Bandera, Mérida, Venezuela from January 2013 to January 2014 and January–February 2015. Each month value represents the records grouped from two visits.
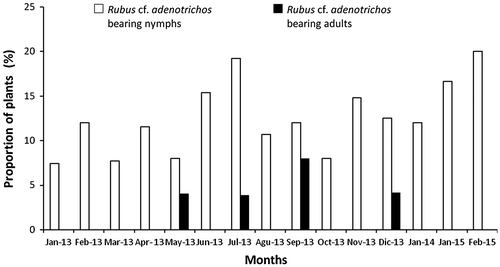
Figure 2. Heniartes stali-Rubus cf. adenotrichos interaction in the study area. (a) A late instar nymph walking on a petiole (note the dense cover of the long stalked glandular trichomes); (b) Molting nymph; (c) Early instar nymph feeding on a caterpillar; and (d) TLC plate of the sticky fluid extracts obtained from different samples.
We inspected 84 individuals of R. cf. adenotrichos in the other additional sites studied. The mean percentage of plants bearing H. stali individuals was 33.23 ± 12 (Figure ). In total, plants bore greater number of nymphs compared to adults (X2 = 4.55; p < 0.05). We found 18 nymphs, seven adult males and one adult female during these surveys.
Study of the insect sticky material source
The sticky materials eluted with the solvent from metatibiae of nymphs, adults, egg covers, and stem exudate of R. cf. adenotrichos showed very similar TLC spot patterns (Figure (d)), thus strongly suggesting them to be the same untransformed material.
TS gathering and manipulation behavior of H. stali
Females of H. stali harvested and manipulated TS during several hours according to the following behavioral patterns:
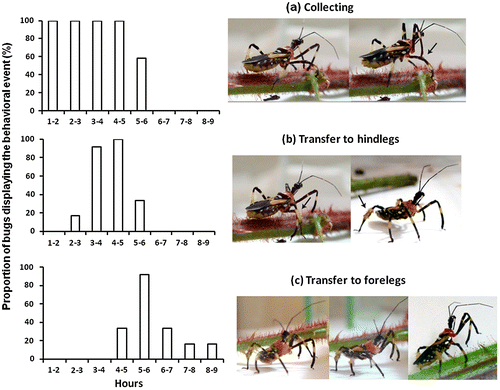
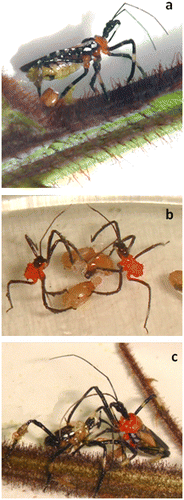
| (1) | TS collecting (Figure (a)): The harvesting of the trichome droplets was the event in which the bugs invested more time. The insects collected TS with the apex of the protibia with brushing-like movements over the trichome tips. After this, the apex of the protibia was thrusted towards the dorsal surface of the mesofemur of the same body side and the area smeared with the TS up to the middle of the mesotibia. These movements were repeated with only one foreleg, or synchronically with both forelegs, during most of the observation period. | ||||
| (2) | TS transfer to hindlegs (Figure (b)): When the mesofemur and mesotibia were heavily coated with the TS, after 2–5 h of harvesting, the bug extended the hindleg to scrape off the accumulated material from the mesofemur and mesotibia. The brush-like hairs of the metatibiae thus became covered with plentiful TS. | ||||
| (3) | TS transfer to forelegs (Figure (c)): Between the fourth and ninth hour after beginning of harvesting, females carried TS accumulated on the metatibia to the midleg by rubbing the tibiae of both legs. Subsequently, TS was dispersed by the mesotibia on the foreleg on each side of the body. Lateral material transfer occurred when the forelegs were rubbed together. Also, insects used forelegs to smear the first segments of their antennae and proboscis. Wing edges and lateral abdominal sections were spread by brushing the metatibiae with the accumulated material. All juvenile stages and adult males displayed the same behavioral events in TS harvesting and storing up. | ||||
Figure 4. Time course of the trichome secretion (TS) harvesting and manipulation behavior by Heniartes stali females (N = 12) (a) From the first minutes and, during several hours, the bugs started to gather the TS from the trichomes with the tips of the forelegs and then they were used for smearing the midlegs (black arrow); (b) After two hours at least, the TS were brushed off from the midleg with the hairy area of the metatibia where it was stored (black arrows); and (c) The transfer to forelegs started four hours after that the collecting had begun, when the hindleg and midleg tibiae were rubbed. Then, the TS was smeared on the foretibia by the mesotibia and a lateral transfer occurred when the forelegs were rubbed together.
We also observed that females smeared portions of TS on the metatibiae with rhythmical upward and downward brushing movements on segments of genitalia during oviposition (Figure (a)). Both hindlegs were used alternatively for this purpose. After a while, a reduced load of TS on the metatibial brush became apparent. We recorded the smearing activity for each egg laid.
Figure 5. Other forms of relocation of Rubus cf. adenotrichos TS displayed by Heniartes stali. (a) A female spreading the TS on the genital area using a hind leg; (b) Newly hatched nymphs collecting the TS from eggs; and (c) A freshly molted male collecting the TS from the exuvia.
The TS collection behavior appeared early in life in H. stali, even in the absence of plants. Newly hatched first instar nymphs picked up TS from the surface of eggs, deposited there earlier by mothers (Figure (b)). The strong TS collection impulse in these insects became evident as well by newly molted late stage nymphs and imagoes gathering residual TS up from front legs of exuviae within the first few hours of their molting (Figure (c)).
Discussion
R. cf. adenotrichos is occupied frequently by H. stali, which the insect uses as a sticky material source
The regular presence of juveniles of H. stali on the blackberry plants suggests that H. stali is a deliberate TS exploiter. Although we were unable to find eggs there in our observations, the occurrence of early first instar nymphs on these plants supports the notion that females seem to use them for oviposition. Further monitoring will be necessary to confirm this hypothesis. Furthermore, we showed that the chemical composition of TS and the sticky material stored in bug legs was very similar, clearly confirming the source and the lack of additional chemical transformation to fulfill the insect’s needs. The yearlong abundance of glandular trichomes in evergreen blackberry plants appears as the key element supporting the plant–insect relationship. That this association may also be nonspecific is suggested by our occasional findings (pers. obs.) of nymphs on Melinis minutiflora grass, leaves and stems of which are covered with a thick layer of glandular trichomes secreting a gummy fluid.[Citation15]
The relationship of certain Harpactorinae with plants is not rare. Other undetermined species of Heniartes have also been observed in the field on plants bearing glandular trichomes such as some Melastomataceae.[Citation16] Thus, trichome fluid use may extend beyond H. stali within the genus Heniartes but further research is needed before conclusions in this regard can be reached. Also in parallel with H. stali, females of the bee killer Apiomerus flaviventris are known to collect glandular trichome exudates from Heterotheca psammophila (Asteraceae), deposit them on the ventral surface of abdomen using their legs, and subsequently transfer them to eggs.[Citation4] Resin collection with the forelegs by Apiomerini Beharus cylindripes (Fabricius), Manicoris rufipes (Fabricius), and Ponerobia bipustulata (Fabricius) also has been documented.[Citation17–19]
H. stali gathers and manipulates with its legs the TS from R. cf. adenotrichos
TS accumulation on hindlegs and transfer to eggs through a well-defined pattern shows the outstanding ability of H. stali in handling this viscous material for several hours. To the best of our knowledge, handling behavioral issues in Neotropical Apiomerini remain undocumented. There is a detailed study [Citation5] for the Nearctic resin bug A. flaviventris, which also included the insect’s body structures involved in resin manipulation. Although some behavioral convergence with H. stali is evident, an important difference between these insects is the final storage site for the collected material. While A. flaviventris stores the resin on the ventral surface of the abdomen, H. stali seems to prefer setal structures of metatibiae. Other behavioral disparities apply to sex and nymphal stage. The authors [Citation5] observed that, as opposed to adult females, A. flaviventris males generally did not gather plant resin. They also noted that although first instar nymphs collected resin from the egg mass after hatching as well as from a resin drop experimentally provided, later instars were rarely observed displaying the resin gathering behavior.
In contrast, males and all the instars of H. stali exhibited strong proneness to gather R. cf. adenotrichos trichome secretion in addition to recovering it from egg covers and exuviae. Extending on these contrasts, we noted that H. stali females smeared the genital area with the TS stored in the hairs of hindtibiae during oviposition, whereas A. flaviventris did so from the material spread over the ventral abdomen. The persistent application of TS over genitalia in females of H. stali suggests that eggs are coated with this material as they emerge through the genital opening. This strategy was interpreted by Eisner et al. [Citation4] as aimed at protecting eggs against ants and microbial pathogens in A. flaviventris, in consonance with Choe and Rust [Citation3] in the case of brittlebush resin. Maternal care was the term used by Forero et al. [Citation5] to describe this behavior on A. flaviventris. They hypothesized that maternal care is likely to occur in genera with dense ventral abdominal setal patches – which enable resin storage – where hypothetic ventral abdominal glands may secrete a substance to prevent the desiccation of the viscous material. We confirmed the occurrence of maternal care in H. stali but, even though we were not able to confirm the presence of abdominal glands, we did substantiate the lack of abdominal setal patches in females, thus adding a new variant to maternal care behavior in Apiomerini.
Other than maternal care, we also report here a previously undescribed TS storage behavior in hind legs and its relocate to fore legs and other body structures by nymphs and males. This suggest that the TS could be used by H. stali to enhance the hunting abilities and deter predators and parasitoids as is suggested for other Apiomerini.[Citation4,5]
Finally, our findings reveal a previously unsuspected interaction between Heniartes and a trichome-bearing plant in the Neotropics. This is a remarkable instance of a predatory insect using a plant product as a resource to, probably, become more effective in hunt-feeding and defense. Additional chemical studies and observations on the prey capture behavior may open avenues to reveal further functions of the trichome secretion collected by H. stali from R. cf. adenotrichos.
Associate Editor: Esteban Suarez
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Consejo de Desarrollo Científico,Humanístico,Tecnológico y de las Artes (CDCHTA) of Universidad de Los Andes, Mérida, Venezuela [grant number C-1773-12-01-B and SE-C-06-14-01].
Acknowledgements
We thank D. Forero for identifying the genus of our subject insect and encouraging our research. We appreciate the help during field work of Maria Pia Calcagno and Francisco López. We also thank H.R. Gil-Santana for kindly providing useful literature on the topic.
References
- Schuh RT, Slater JA. True bugs of the world (Hemiptera:Heteroptera): classification and natural history. New York: Cornell University Press; 1995.
- Gil-Santana HR, Costa LAA, Forero D, et al. Sinopse dos Apiomerini, com chave ilustrada para os géneros (Hemiptera-Heteroptera,Reduviidae,Harpactorinae) [Synopsis of Apiomerinae with illustrated key to the genera (Hemiptera-Heteroptera, Reduviidae, Harpactorinae)]. Pub. Av. Mu. Nac. 2003;97:1–24. Portuguese.
- Choe DH, Rust M. Use of plant resin by a bee assassin bug, Apiomerus flaviventris (Hemiptera: Reduviidae). Ann. Entomol. Soc. Am. 2007;100:320–326.10.1603/0013-8746(2007)100[320:UOPRBA]2.0.CO;2
- Eisner Th, Eisner M, Seigler M. Secret weapons: defenses of insects, spiders, scorpions and other legged creatures. Cambridge (MA): Harvard University Press; 2005.
- Forero D, Choe DH, Weirauch C. Resin gathering in neotropical resin bugs (Insecta: Hemiptera: Reduviidae): functional and comparative morphology. J. Morphol. 2011;272:204–229.10.1002/jmor.v272.2
- Wygodzinsky P. Contribuiçao ao conhecimento do gènero Heniartes Spinola 1837 (Apiomerinae, Reduviidae, Hemiptera) [Contribution to the knowledge of the genus Heniartes Spinola 1837 (Apiomerinae, Reduviidae, Hemiptera)]. Arq. Mus. Nac. 1947;41:11–65. Portuguese.
- Gil-Santana HR, Forero D. Taxonomical and biological notes on neotropical Apiomerini (Hemiptera:Heteroptera: Reduviidae: Harpactorinae). Zootaxa. 2010;2331:57–68.
- Wygodzinsky P. Contribuiçao ao conhecimento do gènero Heniartes Spinola, 1837 e “Agriocleptes” Stal, 1866 (Apiomerini, Reduviidae, Hemiptera) [Contribution to the knowledge of the genus Heniartes Spinola, 1837 and "Agriocleptes" Stal, 1866 (Apiomerini, Reduviidae, Hemiptera)]. Rev. Brasil. Bio. 1953;13:369–380. Portuguese.
- Alice LA, Campbell CS. Phylogeny of Rubus (Rosaceae) based on nuclear ribosomal DNA internal transcribed spacer region sequences. Am. J. Bot. 1999;86:81–97.10.2307/2656957
- Somayeh AJ, Iraj M, Alireza T, et al. Intra-specific variations of Rubus sp. (Rosaceae) in Northern Iran: morphometric analysis and microsatellite markers. J. Bio. & Env. Sci. 2014;5:189–198.
- Mertz C, Cheynier V, Günata Z, et al. Analysis of phenolic compounds in two blackberry species (Rubus glaucus and Rubus adenotrichus) by high-performance liquid chromatography with diode array detection and electrospray ion trap mass spectrometry. J. Agric. Food Chem. 2007;55:8616–8624.10.1021/jf071475d
- Tissier A. Glandular trichomes: what comes after expressed sequence tags? Plant J. 2012;70:51–68.10.1111/j.1365-313X.2012.04913.x
- Fürstenberg-Hägg J, Zagrobelny M, Bak S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013;14:10242–10297.10.3390/ijms140510242
- Durant P, Zambrano E, Arellano E. Aula Ambiental de la Universidad de Los Andes; Edición de la Cátedra Libre de Estudios Ambientales [Environmental classroom of the University of the Andes]. Mérida: Universidad de Los Andes; 2002. Spanish.
- Prates HT, Leite RC, Craveiro AA, et al. Identification of some chemical components of the essential oil from molasses grass (Melinis minutiflora Beauv.) and their activity against cattle-tick (Boophilus microplus). J. Braz. Chem. Soc. 1998;9:193–197.10.1590/S0103-50531998000200013
- Zhang G, Weirauch C. Sticky predators: a comparative study of sticky glands in harpactorine assassin bugs (Insecta: Hemiptera: Reduviidae). Acta Zoologica. 2013;94:1–10.10.1111/azo.2012.94.issue-1
- Gil-Santana HR, Alves RJ. Association between Zelus versicolor (Herrich-Schäffer) (Hemiptera, Reduviidae, Harpactorinae) and Bidens rubifolia Kunth (Asterales, Asteraceae). EntomoBrasilis. 2011;4:30–32.10.12741/ebrasilis.v4i1
- Bérenger JM, Pluot-Sigwalt D. Relations privilégiées de certains Heteroptera Reduviidae prédateurs avec les végétaux. Premier cas connu d'un Harpactorinae phytophage [Privileged relationship of some Heteroptera Reduviidae predators with plants. First known case of a phytophagous harpactorinae]. Comptes Rendus de l'Académie des Sciences - Series III - Sciences de la Vie. 1997;320:1007–1012. French.10.1016/S0764-4469(97)82474-2
- Melo MC, Berkov A, Coscaron MC. Redescription of Manicocoris rufipes (Fabricius 1787), including nymphs I, II, III, and V (Reduviidae: Harpactorinae: Apiomerini), and its association with Clusia fruits. Stud. Neotrop. Fauna Environ. 2005;40:55–64.10.1080/01650520500040538