Abstract
The Atlantic Forest is one of the most diverse and threatened tropical forests in the world, and the Rio Doce valley seems to represent a limit between biotas from its southern and northern portions. In the present study, we investigated the distribution of Kielmeyera (Calophyllaceae), a woody, typically wind-dispersed genus, with a high endemism rate in the Atlantic Forest. The 351 specimens analysed, representing 21 of 22 species of Kielmeyera from the Atlantic Forest, pointed to a complete dissimilarity between species composition in the southern (Rio de Janeiro state) and northern (Bahia state) portions of the forest. However, the Rio Doce valley in Espírito Santo state, rather than being a limit between these two portions, appear to represent a confluent area. The northern boundary of species from the southern portion is the São Mateus basin, whereas the southern boundary of species from the northern portion is at the Litorânea do Espírito Santo basin. Most specimens of Kielmeyera were collected in areas that currently are not remnants of original vegetation and that are not within any conservation unit, suggesting that an imminent loss of biodiversity is taking place. Studies comprising a broad range of functionally and phylogenetically distinct taxa make individual patterns of distribution obscure and overlook the conservation needs for specific groups. Therefore, our study on the distribution of Kielmeyera in the Atlantic forest highlights the importance of biogeographic analyses of less inclusive taxa of a flora.
A Mata Atlântica é uma das florestas tropicais mais diversas e ameaçadas do mundo, e o vale do Rio Doce parece representar um limite entre as biotas de suas porções norte e sul. Neste estudo, nós investigamos a distribuição de Kielmeyera (Calophyllaceae), um gênero lenhoso, tipicamente anemocórico, com alta taxa de endemismo na Mata Atlântica. Os 351 espécimes analisados, representando 21 das 22 espécies de Kielmeyera da Mata Atlântica, indicaram uma completa dissimilaridade entre a composição de espécies nas porções sul (Rio de Janeiro) e norte (Bahia). Entretanto, o vale do Rio Doce, no Espírito Santo, em vez de limite entre as duas porções, parece representar uma área de confluência. O limite norte para as espécies da porção sul é a bacia do Rio São Mateus, ao passo que o limite sul para as espécies da porção norte é a bacia Litorânea do Espírito Santo. A maioria dos registros para o gênero não está em áreas protegidas ou remanescentes de vegetação original, sugerindo que uma perda eminente de sua diversidade já se encontra em andamento. Estudos abrangendo uma ampla gama de táxons funcional e filogeneticamente discrepantes tornam padrões individuais de distribuição obscuros e negligenciam as necessidades de conservação para grupos específicos. Assim, nosso estudo sobre a distribuição de Kielmeyera na Mata Atlântica mostra a importância de análises biogeográficas de táxons menos inclusivos de uma flora.
Palavras-chave:
Introduction
The Atlantic Forest is one of the most diverse tropical forests and one of 34 areas in the world with a high number of endemic species at imminent risk of extinction; as such, protecting its biota is a priority in conservation biology [Citation1]. It is predominantly distributed in Brazil (90%), and also occurs in Argentina and Paraguay [Citation2]. In Brazil, the Atlantic Forest shelters more than 17,000 species of plants (Lista de espécies do Brasil <http://floradobrasil.jbrj.gov.br/>. accessed at 18 May 2015); of which, approximately 45% are endemic [Citation3] and 10% are threatened [Citation4]. This phytogeographic domain consists mainly of wet forests, with high annual rainfall (~2,000 mm/year) and continuous precipitation (>60 mm/month) [Citation5], but also comprises other phytophysiognomies (e.g. ombrophilous dense, ombrophilous open, ombrophilous mixed, deciduous and semideciduous forests, as well as mangroves, restinga and dunes) [Citation6]. Officially, the Atlantic Forest comprises 1,500,000 km², currently scattered in approximately 245,000 fragments, most of which are small (<50 ha) and often composed of secondary forests [Citation7].
As in every biologically rich area, biodiversity is not equally distributed throughout the Atlantic Forest [Citation8,9], and distribution patterns in the domain seem to converge into four main centres of endemism: Pernambuco, southern Bahia, Serra do Mar and the Araucaria forest [Citation10]. Nevertheless, every group of organisms have morphological and ecological features that contribute differently to the reproductive success, dispersal capability and habitat specificity of a species in a certain region; and, together, they affect the potential distribution and abundance of the species [Citation11]. As a result, areas of endemism may vary according to the group: from six for angiosperm epiphytes [Citation12], to as many as 12 for harvestmen (Arachnida), which present high habitat requirement and low vagility [Citation13]. These centres of endemism often represent refuges with high climatic stability, usually less susceptible to extinction events and with high rates of diversification [Citation14,15]. The recognition of these biogeographic patterns, therefore, may help us to understand ecological and evolutionary processes, and so encourage conservation efforts [Citation16].
In the Atlantic Forest, there is a predominance of species with fleshy fruits, dispersed by animals (zoochory), whereas wind dispersal (anemochory) is poorly represented, comprising usually less than 15% of species [Citation17–19]. Factors affecting wind dispersal are different from those affecting animal dispersal [Citation20] and wind-dispersed trees tend to reach smaller dispersal distances in tropical wet forests when compared to trees dispersed by animals, especially birds and mammals [Citation21]. Therefore, anemochoric species may display biogeographic patterns that are different from most species in the Atlantic Forest; and thus, be under distinct threats and may need specific requirements when compared to those applied to the Atlantic Forest as a whole. In the present study, we investigated the distribution pattern of a typically anemochoric genus, Kielmeyera Saddi, in the Atlantic Forest to identify centres of diversity and endemism of the genus and potential geographic barriers for the species. As a wind-dispersed group, we expect that the distribution pattern of Kielmeyera will diverge from those recovered from most groups in the Atlantic Forest [e.g. Citation10, and references therein].
Methods
Kielmeyera consists of trees, shrubs and subshrubs, with staminate or bisexual flowers pollinated by bees, and fruits that are woody capsules with winged seeds [Citation22] (Figure ). The genus occurs predominantly in Brazil; only 2 of the 48 species of Kielmeyera do not occur in this country. The genus shows a high rate of endemism in the Atlantic Forest, where 22 out of the 46 Brazilian species occur [Citation23]. Most species from this domain are endemic (18 species, corresponding to almost 40% of all species of the genus), 7 are rare [Citation24] and 4 threatened [Citation25].
Figure 1. Representatives of Kielmeyera (Calophyllaceae) from the Atlantic Forest. (A) K. albopunctata: branch with flowers; (B, C) K. argentea: (B) shrub in restinga―quaternary sandy coastal plain―vegetation, Salvador, Bahia state, (C) opened fruit, showing seeds; (D, E) K. elata: (D) tree in a wet forest, Ilhéus, southern Bahia, (E) branch with leaves; (F, G) K. ferruginosa: (F) trunk with bark partially removed to show the orange latex, (G) a population in a flooded arboreal restinga, Una, southern Bahia; (H) K. membranacea: branch with a flower; (I) K. marauensis: branch with flowers; (J, K) K. neglecta: (J) tree in a wet forest, Serra Grande, southern Bahia; (K) branch with flowers; (L, M) K. reticulata (L) branch with flowers, (M) fruit; (N, O) K. rugosa: trunk with bark partially removed to show the yellowish latex, (N) branch with a flower, in shrubby tabuleiro, a semideciduous coastal forest, northern Bahia. Photos by F.S.E. Santo (A, H, J); A.P.B. Santos (B–G, L, N, O); I.S. Abreu (M); D.N. Carvalho (I, K).
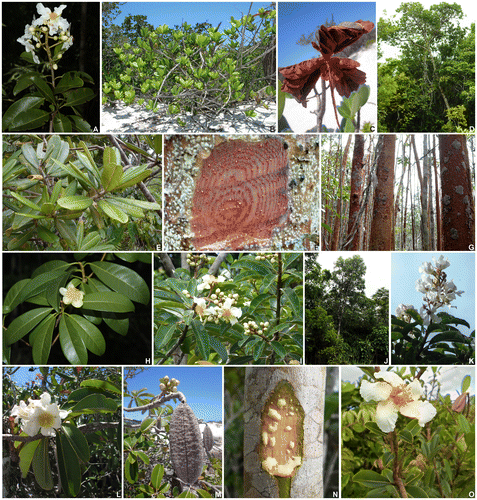
Based on a database of approximately 2,000 collections of Kielmeyera deposited at the main Brazilian herbaria and two international herbaria (MO and NY- USA), we built a matrix of the geographic distribution of specimens from the Atlantic Forest with reliable identification (identified or confirmed by specialists on the group). This matrix consisted of 351 recorded specimens, which represented 21 species of the genus (Table ). Kielmeyera divergens was not included in our analyses because its locality in Espírito Santo is unknown [Citation26]. GPS coordinates of collections were obtained from herbarium labels and confirmed or recovered with Google Earth, using the GeoLoc tool (http://splink.cria.org.br/geoloc).
Table 1. Number of records from the river basins of the Atlantic Forest (southwards) per species of Kielmeyera.
Spatial and cluster analyses were performed in Biodiverse, version 0.19 [Citation27], using grids of 0.5° × 0.5° (~50 × 50 km) and one neighbour cell. We calculated sampling (number of collections), richness (number of species), corrected weighted endemism (see [Citation28,29]) and diversity (Shannon-Wiener index) per cell. Cluster analysis was performed using Sorensen-Dice dissimilarity coefficient. We also overlapped Kielmeyera records onto areas of remnants [Citation30] and conservation units [Citation31] in the Atlantic Forest. To check whether results have been strongly biased by sampling effort, we conducted a correlation analysis between sampling and richness in SAM (Spatial Analyses in Macroecology 3.1) [Citation32].
Results
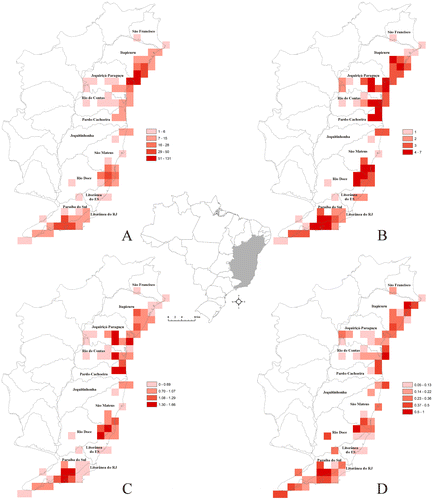
Richness and sampling were not significantly correlated (Spearman coefficient: r = 0.146, p = 0.627). Sampling efforts were mainly concentrated in northern Bahia, between the Itapicuru and Jequiriçá-Paraguaçu basins, at the boundary of the Todos os Santos Bay (Figure (A)), whereas the richest areas are scattered in the Itapicuru (6 species), Jequiriçá-Paraguaçu, Rio de Contas and Pardo-Cachoeira (5 species each) basins in Bahia state, the Rio Doce (7 species) and São Mateus (6 species) basins in Espírito Santo state and the Paraíba do Sul (7 species) and Litorânea do Rio de Janeiro (5 species) basins in Rio de Janeiro state (Figure (B); Table ). Areas with the highest diversity were distributed in three centres: in Bahia, scattered among the Jequiriçá-Paraguaçu, Rio de Contas and Pardo-Cachoeira basins; in Espírito Santo, in the Rio Doce basin; and in Rio de Janeiro state, between the Litorânea do Rio de Janeiro and Paraíba do Sul basins (Figure (C)). Finally, areas with the highest corrected weighted endemism were located in the northern Itapirucu basin, at the junction among the Jequiriçá-Paraguaçu, Rio de Contas and Pardo-Cachoeira basins, in the Rio Doce basin and in the Paraíba do Sul basin (Figure (D)).
Figure 2. Aspects of Kielmeyera distribution in Atlantic Forest on a grid of 0.5° × 0.5° cells, showing the river basins along the coast. (A) sampling; (B) richness; (C) H’ diversity; (D) corrected weighted endemism.
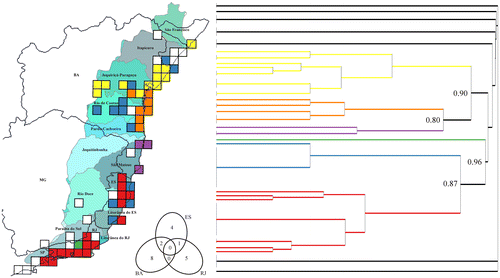
Cluster analysis showed the species of Kielmeyera distributed in 67 squares, with higher heterogeneity northward. Bahia, with 10 species, and Rio de Janeiro, with six, did not share any species of Kielmeyera in the Atlantic Forest. Espírito Santo, with seven species, shares one species with Rio de Janeiro and two with Bahia, presenting some areas that are more similar to areas in Bahia, and others to areas in Rio de Janeiro (Figure ). Alagoas and Sergipe states have one species each and both are shared with Bahia, whereas São Paulo and Minas Gerais states have one exclusive species each.
Figure 3. Cluster analysis (Sorensen dissimilarity coefficient) of the composition of Kielmeyera in Atlantic Forest, on a grid of 0.5° × 0.5° cells. Colours in the dendogram correspond to those on the map (black lines correspond to white cells) and node labels correspond to the distance (dissimilarity) between clusters. The Venn diagram shows the number of species in the states of Bahia (BA), Espírito Santo (ES) and Rio de Janeiro (RJ). On the map, black lines indicate states and grey lines indicate river basins (in different colours only to favour visualization).
Most specimens of Kielmeyera (57%) were collected in areas that are not currently remnants or in which the remnants are extremely small (<3 ha) for holding healthy populations over lengthy periods. Only 17% of specimens were collected in Conservation Units and, possibly, three threatened species of Kielmeyera are not protected in any of them: K. ferruginosa [Citation33], K. rufotomentosa [Citation26] and K. rupestris [Citation34], with the former species only recently discovered in an area that is already being disturbed [Citation33].
Discussion
Apart from the Itapicuru and Jequiriçá-Paraguaçu basins (northern Bahia), the other areas with a higher richness of Kielmeyera coincide with centres of plant diversity and endemism in the Atlantic Forest: Rio de Contas and Pardo-Cachoeira basins (southern Bahia) [Citation35–37], São Mateus and Rio Doce basins (northern Espírito Santo) [Citation36–38], and Litorânea do Rio de Janeiro and Paraíba do Sul basins in Rio de Janeiro state [Citation35–37]. This pattern does not seem to be a sampling artefact, as verified in other works on plants [Citation35,36,38], because sampling and richness in our study are not significantly correlated. The sharp division between Kielmeyera species composition in Bahia and Rio de Janeiro mirror the existence of two centres of diversity that possibly worked as refuges during the Quaternary [Citation14]. In the northern portion, southern Bahia is an important centre of endemism for angiosperms [Citation35–39], whereas in the southern portion, the Serra do Mar (sensu [Citation40]) is known for its high concentration of endemic species from different groups of animals (birds, mammals and butterflies) [Citation41–44]. Although Porto et al. [Citation45] suggested a large refuge in the southern portion of the Atlantic Forest, including also northeastern São Paulo and southeastern Minas Gerais, Carnaval et al. [Citation46] suggested that endemisms in this region might have resulted from its current climatic heterogeneity and not necessarily because of past climatic stability.
Rivers can work as geographic barriers, contributing to diversification and helping to mould the distribution pattern of several organisms [e.g. Citation47, Citation48]. Carnaval and Moritz [Citation14] pointed out the São Francisco and Rio Doce rivers as the most determinant biogeographic barriers in the Atlantic Forest. Many studies with plants [e.g. 39], and animals [12 and references therein] emphasized the importance of the Rio Doce in dividing the Atlantic Forest biota into northern and southern portions [Citation49]. Nevertheless, for some groups (mammals [Citation43], arthropods, snakes and frogs, but also plants, Bromeliaceae and Poaceae [Citation50]) this division seems to occur in the Paraíba do Sul valley along with the Mantiqueira mountain range and for others (lizards [Citation48] and birds [Citation51]) in the Jequitinhonha valley. The distribution of Kielmeyera is delimited in the north by the São Francisco river and in the south by the Paraíba do Sul river. The Rio Doce basin in Espírito Santo emerges as a transitional area, presenting areas that are more similar to the northern portion and areas that are more similar to the southern portion of the Atlantic Forest, result similar to that revealed by Carnaval et al. [Citation46]. Its similarity with the northern portion is defined by the occurrence of K. albopunctata, which is also distributed in seasonal dry forests, whereas its similarity with the southern portion is defined by the distribution of K. membranacea along the coast, from Rio de Janeiro to Espírito Santo (Table ).
Since Kielmeyera seeds are dispersed preferentially during the dry season [Citation52,53] and usually only reach about 10 m from the mother plant [Citation54], or even less in wet forests, where wind incidence is lower, geographic and ecological barriers or physiological constraints that affect the distribution of species in this genus may contrast with those presented by most groups from the Atlantic Forest. Although Rio de Janeiro and Bahia do not share any species of Kielmeyera from the Atlantic Forest, the distribution of one species from Rio de Janeiro exceeds the Rio Doce basin, having its northern limit at the São Mateus basin, whereas the distribution of two species from Bahia exceed the Rio Doce basin, reaching their southern limit at the Litorânea do Espírito Santo basin (Figure ). Therefore, the Rio Doce basin, rather than being a boundary between the northern and southern Atlantic Forests, seems to represent a confluent area for Kielmeyera. Despite its biogeographic importance, this basin has been highly disturbed and, more recently (November 2015), was the subject of a huge environmental disaster caused by the rupture of a barrage that released millions of m3 of mining waste into the Rio Doce. This tragedy destroyed approximately 1,500 hectares of vegetation along 77 km of watercourses, including permanent preservation areas [Citation55].
The high number of endemic species of Kielmeyera in the Atlantic Forest and the reduced areas of primary vegetation following a long history of human occupation make the conservation of these species a priority. Currently, only 12% of the Atlantic Forest retains its original vegetation [Citation7]. Although Bahia shelters the highest number of species of Kielmeyera in the Atlantic Forest, it shows the lowest rate of collections in remnants (34.6% vs. ~ 65% in Espírito Santo and Rio de Janeiro) and Conservation Units (12% vs. ca. 25% in Espírito Santo and 20% in Rio de Janeiro), which is certainly caused by the rapid loss of native vegetation and the small area that is under legal protection, which represents only 10.7% of the state [Citation56].
Centres of diversity and endemism denote important biogeographic patterns and can be used to guide conservation policies. However, the distribution of each group is driven by specific historical and ecological factors. Our results show that the distribution boundaries of Kielmeyera in the Atlantic Forest appear to be at the São Mateus and Litorânea do Espírito Santo basins, and that the Rio Doce basin emerges as a confluent area between northern and southern floras, rather than as a limit between them, as recurrently found for several groups of plants [Citation39] and animals [Citation10,51,57]. The biogeographic pattern revealed here based on Kielmeyera may have been caused by (1) wind dispersal, as most species of plants from the Atlantic Forest are dispersed by animals, (2) intrinsic constraints that may impose different climatic and ecologic limits on this genus and/or also, (3) a recent history of dispersal from floristic refuges in the northern and southern portions of the Atlantic Forest, resulting in a yet incomplete occupation of the species’ potential distribution.
The high rate of Kielmeyera species endemic to the Atlantic Forest make their protection essential for preserving most of the generic diversity. Therefore, our study calls attention to the importance of biogeographic analyses with specific groups from a flora since combining taxa that are functionally distinct and phylogenetically unrelated may mask particular patterns of distribution and neglect taxa that also merit urgent attention.
Associate Editor: Nora Oleas.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico [Universal n° 482988/2013-4]; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
References
- Mittermeier RA, Gil PR, Hoffman M, et al. Hotspots revisited: earth’s biologically richest and most endangered terrestrial ecoregions. Chicago (IL): University of Chicago Press; 2004. p. 392.
- Galindo-Leal C, Câmara IG. Mata Atlântica: biodiversidade, ameaças e perspectivas [Atlantic Forest: biodiversity, threats and perspectives]. Belo Horizonte: Fundação SOS Mata Atlântica/Conservação Internacional; 2005. 472 p.
- Stehmann JR, Forza RC, Salino A, et al., editors. Plantas da Floresta Atlântica [Plants of the Atlantic Forest]. Rio de Janeiro: Jardim Botânico do Rio de Janeiro; 2009. 500 p.
- Biodiversitas [Internet]. Lista da flora brasileira ameaçada de extinção [List of Brazilian flora threatened with extinction]. Fundação Biodiversitas; 2005 [cited 2015 Jan 05]. Available from: http://www.biodiversitas.org.br/florabr/
- Underwood EC, Olson D, Hollander AD, et al. Ever-wet tropical forests as biodiversity refuges. Nat Clim Chang. 2014;4:740–741.10.1038/nclimate2351
- Joly CA, Aidar MPM, Klink CA, et al. Evolution of the Brazilian phytogeography classification systems: implications for biodiversity conservation. Ciênc Cult. 1999;51(5/6):331–348.
- Ribeiro MC, Metzger JP, Martensen AC, et al. The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation Biol Conserv. 2009;142:1141–1153.10.1016/j.biocon.2009.02.021
- Gaston KJ. Global patterns in biodiversity. Nature. 2000;405:220–227.10.1038/35012228
- Myers N, Mittermeier RA, Mittermeier CG, et al. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858.10.1038/35002501
- DaSilva MB, Pinto-da-Rocha R. A história biogeográfica da Mata Atlântica: opiliões (Arachnida) como modelo para sua inferência [The biogeographical history of the Atlantic Forest: harvestmen (Arachnida) as a model for its inference]. In: Carvalho CJB, Almeida EAB, editors. Biogeografia da América do Sul. Padrões & Processos. São Paulo: Roca; 2011. p. 221–238.
- Krebs CJ. Ecology: the experimental analysis of distribution and abundance. 6th ed. San Francisco (CA): Benjamin Cummings; 2009. p. 655.
- Menini-Neto L, Furtado SG, Zappi DC, et al. Biogeography of epiphytic angiosperms in the Brazilian Atlantic Forest, a world biodiversity hotspot. Braz J Bot. 2015;39(1):261–273.
- DaSilva MB, Pinto-da-Rocha R, DeSouza AM. A protocol for the delimitation of areas of endemism and the historical regionalization of the Brazilian Atlantic rain forest using harvestmen distribution data. Cladistics. 2015;31(6):692–705. DOI:10.1111/cla.12121.
- Carnaval AC, Moritz C. Historical climate modelling predicts patterns of current biodiversity in the Brazilian Atlantic Forest. J Biogeogr. 2008;35:1187–1201.10.1111/jbi.2008.35.issue-7
- Jetz W, Rahbek C, Colwell RK. The coincidence of rarity and richness and the potential signature of history in centres of endemism. Ecol Lett. 2004;7:1180–1191.10.1111/ele.2004.7.issue-12
- Orme CD, Davies RG, Burgess M, et al. Global hotspots of species richness are not congruent with endemism or threat. Nature. 2005;436(7053):1016–1019.10.1038/nature03850
- Morellato LPC, Leitão-Filho HF. Padrões de frutificação e dispersão na Serra do Japi [Fruiting and dispersion patterns in Serra do Japi]. In: Morellato LPC, editor. História Natural da Serra do Japi: Ecologia e preservação de uma área florestal no sudeste do Brasil [Natural History of Serra do Japi: Ecology and preservation of a forest area in southeastern Brazil]. Campinas: UNICAMP/FAPESP; 1992. p. 112–140.
- Almeida-Neto M, Campassi F, Galetti M, et al. Vertebrate dispersal syndromes along the Atlantic Forest: broad-scale patterns and macroecological correlates. Global Eco Biog. 2008;17(4):503–513.10.1111/j.1466-8238.2008.00386.x
- Reznik G, Pires JPA, Freitas L. Efeito de bordas lineares na fenologia de espécies arbóreas zoocóricas em um remanescente de Mata Atlântica [Effect of linear edges in the phenology of zoochoric tree species in a remnant of Atlantic Forest]. Acta Bot Bras. 2012;26(1):65–73.10.1590/S0102-33062012000100008
- van der Pijl L. Principles of dispersal in higher plants. 3rd ed. Berlin: Springer-Verlag; 1982. 214 p.10.1007/978-3-642-87925-8
- Clark CJ, Poulsen JR, Bolker BM, et al. Comparative seed shadows of bird-, monkey-, and wind-dispersed trees. Ecol. 2005;86(10):2684–2694.10.1890/04-1325
- Santos APB, Espírito Santo FS, Rapini A. Flora da Bahia: Calophyllaceae. [Flora of Bahia: Calophyllaceae]. Sitient. Série Ciên Biol. 2015;15:1–27. DOI:10.13102/scb884.
- Bittrich V, Trad RJ, Cabral F, et al. Calophyllaceae [Internet]. In: Lista de espécies da flora do Brasil [List of species of the Brazilian flora]. Jardim Botânico do Rio de Janeiro; 2015 [cited 2015 Jan 08]. Available from: http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB121875
- Bittrich V, Rodrigues WA, et al., editors. Plantas raras do Brasil [Rare plants from Brazil]. Belo Horizonte: Conservação Internacional; 2009. p. 139–141.
- Coelho MAN, Valente ASM, Maurenza D, et al. Clusiaceae. In: Martinelli G, Moraes MA, editors. Livro vermelho da flora do Brasil [Red book of Brazilian flora]. Rio de Janeiro: Andrea Jakobsson Estúdio; 2013. p. 448–450.
- Saddi N. Novas espécies de Kielmeyera Martius (Guttiferae) do Sudeste Brasileiro [New species of Kielmeyera Martius (Guttiferae) from Southeastern Brazil]. Rodriguésia. 1984;36(60):59–64.
- Laffan SW, Lubarsky E, Rosauer DF. Biodiverse, a tool for the spatial analysis of biological and related diversity. Version 0.15. Ecography. 2010;33:643–647.10.1111/eco.2010.33.issue-4
- Laffan SW, Ramp D, Roger E. Using endemism to assess representation of protected areas – The family Myrtaceae in the greater Blue Mountains world heritage area. J Biogeogr. 2012;40:570–578.
- Bitencourt C, Rapini A. Centres of endemism in the Espinhaço range: identifying cradles and museums of Asclepiadoideae (Apocynaceae). Syst Biodivers. 2013;11(4):525–536.10.1080/14772000.2013.865681
- SOS Mata Atlântica. Atlas dos remanescentes florestais da Mata Atlântica, período 2012-2013 [SOS Atlantic Forest. Atlas of forest remnants of Atlantic Forest, period 2012–2013]. São Paulo: Fundação SOS Mata Atlântica/Instituto Nacional de Pesquisas Espaciais; 2014. 61 p.
- MMA [Internet]. Download de dados geográficos: Unidades de conservação [Geographical data download: conservation units]; 2015 [cited 2015 Jan 23]. Available from: http://mapas.mma.gov.br/i3geo/datadownload.htm15
- Rangel TF, Diniz-Filho JAF, Bini LM. SAM: a comprehensive application for spatial analysis in macroecology. Ecography. 2010;33:1–5.
- Santos APB, Trad RJ, Do Espírito Santo FS, et al. Kielmeyera ferruginosa (Calophyllaceae), a new species from the Atlantic Forest, southern Bahia, Brazil. Phytotaxa. 2015;221(3):288–294.10.11646/phytotaxa.221.3
- Duarte AP. Duas novas espécies da flora dos estados do Espírito Santo e Goiás [Two new species of Espírito Santo and Goiás state floras]. Arquivos do Jardim Botânico do Rio de Janeiro. 1973;19:217–222.
- Murray-Smith C, Brummitt NA, Oliveira-Filho AT, et al. Plant diversity hotspots in the Atlantic coastal forests of Brazil. Conserv Biol. 2009;23(1):151–163.10.1111/cbi.2008.23.issue-1
- Werneck MS, Sobral MEG, Rocha CTV, et al. Distribution and endemism of angiosperms in the Atlantic Forest. Natureza & Conservação. 2011;9(2):188–193.10.4322/natcon.2011.024
- Thomas WW, Carvalho AMV, Amorim AMA, et al. Plant endemism in two forests in southern Bahia. Brazil Biodivers Conserv. 1998;7:311–322.10.1023/A:1008825627656
- Giaretta A, de Menezes LFT, Peixoto AL. Diversity of Myrtaceae in the southeastern Atlantic Forest of Brazil as a tool for conservation. Braz J Bot. 2015;38:175–185.10.1007/s40415-014-0121-y
- Prance GT. Forest refuges: evidence from woody angiosperms. In: Prance GT, editor. Biological diversification in the Tropics. New York (NY): Columbia University Press; 1982. p. 137–158.
- Silva JMC, Casteleti CHM. Estado da biodiversidade da Mata Atlântica brasileira [Biodiversity status of the Brazilian Atlantic Forest]. In: Galindo-Leal C, Câmara IG, editors. Mata Atlântica: Biodiversidade, ameaças e perspectivas. [Atlantic Forest: biodiversity, threats and perspectives]. Belo Horizonte: Fundação SOS Mata Atlântica/Conservação Internacional; 2005. p. 43–59.
- Collar NJ, Wege DC, Long AJ. Patterns and causes of endangerment in the new world avifauna. Ornit Monog. 1997;48:237–260.10.2307/40157536
- Manne LL, Brooks TM, Pimm SL. Relative risk of extinction of passerine birds on continents and islands. Nature. 1999;399:258–261.
- Costa LP, Leite YLR, Fonseca GAB, et al. Biogeography of South American forest mammals: endemism and diversity in the Atlantic Forest. Biotropica. 2000;32(4b):872–881.10.1111/btp.2000.32.issue-4b
- Brown KS, Freitas AVL. Atlantic Forest butterflies: indicators for landscape conservation. Biotropica. 2000;32:934–956.10.1111/btp.2000.32.issue-4b
- Porto TJ, Carnaval AC, da Rocha PLB. Evaluating forest refugial models using species distribution models, model filling and inclusion: a case study with 14 Brazilian species. Divers Distrib. 2013;19:330–340.10.1111/ddi.2013.19.issue-3
- Carnaval AC, Waltari E, Rodrigues MT, et al. Prediction of phylogeographic endemism in an environmentally complex biome. Proc R Soc B. 2014;281:20141461.10.1098/rspb.2014.1461
- Moritz C, Patton JL, Schneider CJ, et al. Diversification of rainforest faunas: an integrated molecular approach. Annu Rev Ecol Syst. 2000;31:533–563.10.1146/annurev.ecolsys.31.1.533
- Pellegrino KCM, Rodrigues MT, Waite AN, et al. Phylogeography and species limits in the Gymnodactylus darwinii complex (Gekkonidae, Squamata): genetic structure coincides with river systems in the Brazilian Atlantic Forest. Biol J Linn Soc. 2005;85:13–26.10.1111/j.1095-8312.2005.00472.x
- Fiaschi P, Pirani JR. Review of plant biogeographic studies in Brazil. J Syst Evol. 2009;47(5):477–496.10.1111/j.1759-6831.2009.00046.x
- Sigrist MS, Carvalho CJB. Detection of areas of endemism on two spatial scales using Parsimony analysis of endemicity (PAE): the Neotropical region and the Atlantic Forest. Biota Neotrop. 2008;8(4):33–42.
- Cabanne GS, Santos FR, Miyaki CY. Phylogeography of Xiphorhynchus fuscus (Passeriformes, Dendrocolaptidae): vicariance and recent demographic expansion in southern Atlantic Forest. Biol J Linn Soc. 2007;91:73–84.10.1111/j.1095-8312.2007.00775.x
- Oliveira PE, Silva JCS. Reproductive biology of two species of Kielmeyera (Guttiferae) in cerrados of Central Brazil. J Trop Ecol. 1993;9:67–79.10.1017/S0266467400006970
- Ranieri BD, Negreiros D, Lana TC, et al. Fenologia reprodutiva, sazonalidade e germinação de Kielmeyera regalis Saddi (Clusiaceae), espécie endêmica dos Campos Rupestres da Cadeia do Espinhaço, Brasil [Reproductive phenology, seasonality and germination of Kielmeyera regalis Saddi (Clusiaceae), a species endemic to the rupestrian grasslands of Espinhaço Range, Brazil]. Acta Bot Bras. 2012;26(3):632–641.10.1590/S0102-33062012000300012
- Oliveira PEAM. Biologia de reprodução de espécies de Kielmeyera (Guttiferae) de Cerrados de Brasília, DF [Reproductive biology of Kielmeyera (Guttiferae) species from the Cerrado of Brasília, DF] [dissertation]. Campinas (SP): Universidade Estadual de Campinas; 1986. 95 p.
- IBAMA. Laudo técnico preliminar: Impactos ambientais decorrentes do desastre envolvendo o rompimento da barragem de Fundão, em Mariana, Minas Gerais [Preliminary technical report: environmental impacts of the disaster involving the disruption of Fundão dam in Mariana, Minas Gerais]. Brasília: Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis; 2015. 38 p.
- SEMA [Internet]. Unidades de conservação: Bahia [Conservation units: Bahia]. Secretaria do Meio Ambiente - Governo do Estado da Bahia; 2007 [cited 2016 Apr 14]. Available from: http://www2.meioambiente.ba.gov.br/pdf/Mapasuniconservacao.pdf
- Thomé MTC, Zamudio KR, Giovanelli JGR, et al. Phylogeography of endemic toads and post-Pliocene persistence of the Brazilian Atlantic Forest. Mol Phylogenet Evol. 2010;55:1018–1031.10.1016/j.ympev.2010.02.003
