Abstract
We describe for the first time the vocalization of Scinax haddadorum from its type locality. We also aim to establish an acoustic diagnosis for this species in comparison with the morphologically similar Scinax rupestris, for which we also describe additional note types of its vocalization that were not previously reported. The advertisement call of S. haddadorum consists of a single type of relatively short (<200 ms on average), multipulsed note and the call dominant frequency is restricted mostly to low frequency values (ca. 1.5 kHz), whereas the advertisement call of S. rupestris consists of a single type of relatively long (>450 ms on average), multipulsed note, and the call dominant frequency may be contained within two distinct frequency bands. Advertisement calls across Scinax species exhibit distinctive patterns both in time and frequency structures that should be described in more detail in future studies, since we believe that they might be of help in the recognition and delimitation of species in this group of Neotropical treefrogs.
Introduction
The genus Scinax Wagler, 1830 is one of the most species rich in Hylidae, and had its monophyly corroborated by means of morphological and molecular evidence [Citation1–3]. Species of this genus are widely distributed throughout the Neotropics, ranging from Mexico to Uruguay and northern Argentina [Citation1–4]. The recently described Scinax haddadorum Araujo-Vieira, Valdujo & Faivovich, 2016 is a medium-sized species that is only known from a few sites in the municipality of Barra do Garças (State of Mato Grosso, mid-western Brazil). According to the species description, S. haddadorum is morphologically similar to Scinax rupestriss Araujo-Vieira, Brandão & Faria, 2015, a species that is also known only from its type locality (Chapada dos Veadeiros, State of Goiás, central Brazil). During fieldwork at the type locality of both Scinax species, we recorded their vocalizations, which are herein described. The vocalization of S. haddadorum is described for the first time, as well as note types in the vocalization of S. rupestris that were not previously reported in the species original description. Thus, given the morphological resemblance between S. haddadorum and S. rupestris, we also aim to establish an acoustic diagnosis between these both species.
Material and methods
Fieldwork was conducted at Parque Estadual da Serra Azul (15°85′07.67″S, 52°27′08.08″W, 553 m above sea level), Municipality of Barra do Garças, State of Mato Grosso, mid-western Brazil (further details of the area in Andrade et al. [Citation5]), and in the Municipality of Alto Paraíso de Goiás (14°11′20.01″S, 47°37′22.79″W, 1153 m above sea level), Chapada dos Veadeiros, State of Goiás, central Brazil. Species identifications were based on the original descriptions of S. haddadorum [Citation6] and S. rupestris [Citation7].
Specimens from Barra do Garças (Figure (a)) were identified as S. haddadorum (Araujo-Vieira et al. [Citation6]) based on the following traits: (1) males with moderate size (adult male SVL 31.7–34.1 mm; 33.0 ± 0.7; n = 8); (2) snout acuminate in dorsal view and rounded in lateral view; (3) a nuptial pad that covers the medial margin of Metacarpal I, which obscures the posterior and outer margin of the inner metacarpal tubercle; (4) dorsum with round and irregularly-shaped dark blotches on a dark or light gray, or dark brown background color; (5) hidden portions of thighs and shanks with irregularly-shaped dark blotches on a yellow background color.
Figure 1. (a) Adult male topotype of S. haddadorum (AAG-UFU 1313: SVL 31.7 mm) from Parque Estadual da Serra Azul, Barra do Garças, State of Mato Grosso, western Brazil. (b) Adult male topotype of S. rupestris (AAG-UFU 3334: SVL 27.4 mm) from Alto Paraíso de Goiás, State of Goiás, central Brazil.

Specimens from Alto Paraíso de Goiás (Figure (b)) were identified as S. rupestris (Araujo-Vieira et al. [Citation7]) based on the following traits: (1) relatively small size (adult male SVL 23.6–27.4 mm; 25.3 ± 1.4; n = 6); (2) dorsal coloration consisting of irregularly-shaped blotches and/or spots with variable extent with no distinctive dorsal color patterns of longitudinal stripes, longitudinally arranged blotches, or spots; (3) expanded, median subgular vocal sac; and (4) advertisement call composed of one type of long-lasting (334–708 ms) multipulsed (11–18) note.
Calls of six males of S. haddadorum and four males of S. rupestris were recorded using digital recorders (Marantz PMD 671, Boss BR 864, M-audio Microtrack II) set at sampling rates of 44.1 or 48.0 kHz and at a sample size of 16 bits (mono WAVE file format) coupled to Sennheiser K6/ME67 (Marantz and Boss) and K6/ME66 (Microtrack) directional microphones. Calls were analyzed using Raven Pro 1.5 [Citation8] with the following settings: window type = Hann, window size = 256 samples; 3 dB filter bandwidth = 248 or 270 Hz; overlap (locked) = 85 or 90%; hop size = 0.54, 0.59 or 0.79 ms, DFT size = 1024 samples, grid spacing = 43.1 or 46.9 Hz. All other settings followed the software default values. Temporal traits were measured directly from the oscillogram; the dominant frequency was obtained through ‘Peak Frequency’ measurement function; call rise time was obtained through ‘Peak Time’ measurement function. See Table for acoustic terminology and definitions. Sound figures were made in the See wave package version 1.7.6 [Citation9], R platform version 3.2.3 [Citation10], using the following settings: window = Hanning, overlap = 85% and FFT = 256 samples. Specimens and sound files are housed in the Coleção de Anfíbios do Museu de Biodiversidade do Cerrado, Universidade Federal de Uberlândia (AAG-UFU), Uberlândia, Minas Gerais, Brazil, under the following accession numbers: S. haddadorum: AAG-UFU 1312–1314, 1324–1327, and 5600; S. rupestris: AAG-UFU 3333–3335, and 3346–3348. Analyzed sound files are listed in Appendix 1.
Table 1. Acoustic definitions and terminology employed in the present study.
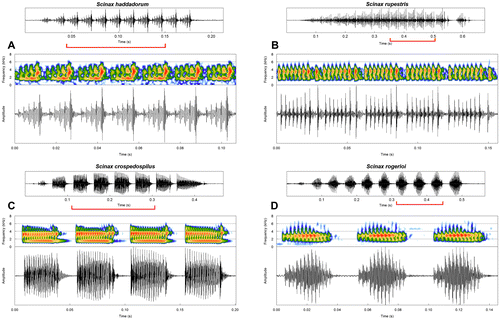
We arbitrarily adopted the straightforward definition for the advertisement call of both species as a single quasi-periodic pulse train (sensu Littlejohn [Citation11]) to facilitate acoustic comparisons among Scinax species, even though we observed regular amplitude modulations (AMs) within the so-called ‘pulse unit’ for the advertisement calls of both studied species, and some other Scinax species (Figure ) (see also Magrini and Giaretta [Citation12]; Magrini et al. [Citation13]; Nunes et al. [Citation14]). It is worth pointing out that there are not any available studies for the genus, in which homologies between acoustic units, such as call, note, and pulse, might be established based on the mechanisms of sound production (see Martin [Citation15,16]; Gans [Citation17]). For this reason, we only classified the ‘within-pulse amplitude modulations’ as pulse sub-units instead of proposing an updated terminology for the AM patterns in Scinax while additional information on this matter is obtained.
Figure 2. Amplitude modulation patterns of the advertisement calls of four species of Scinax. Oscillograms of one call followed by detailed waveforms and spectrograms are portrayed. Fine-scale figures were obtained from oscillogram sections delimited by red brackets for each species. (a) An 11-pulse call of S. haddadorum with regular five-pulse sub-units within each one of the seven pulses detailed in the waveform; sound file: Scinax_haddadorumBarraGarcasMT1fFSA_AAGb; (b) a 17-pulse call of S. rupestris with regular eight- to nine-pulse sub-units within each one of the five pulses detailed in the waveform; sound file: Scinax_rupestrisVeadGO3aCSB_AAGm671; (c) an 8-pulse call of Scinax crospedospilus with regular multipulsed sub-units within each one of the four pulses detailed in the waveform; sound file: Scinax_crospedospilusChiadorMG1bTRC_AAGmt; (d) a 10-pulse call of Scinax rogerioi with regular multipulsed sub-units within each one of three pulses detailed in the waveform; sound file: Scinax_rogerioi_Veadeiros_GO_1BFVT_mct.
Results
S. haddadorum
At Parque Estadual da Serra Azul, males were heard calling along a forest rocky-bottom and clearwater mountain stream (2–5 m wide, 0.5–1.0 m deep). Males were calling in a portion of the stream with scattered bushes, steep terrain and waterfall background noise. Males called from 2 to 5 m from the margins of the stream and at a maximum height of 1 m from the ground. We visited this site five different nights during the summer from October to February (breeding season of most frogs of the region) in five different years and we only found males in calling activity on 15 November 2012 and on 10 November 2016.
The vocalization (n = 6 recorded males) consists of an advertisement call and a second note type. The advertisement call (n = 88 calls; Table , Figure ) consists of a multipulsed note with a progressive increase in amplitude until the third or fourth pulse, when it reaches a plateau that is sustained along the note (Figure (a)), or until the penultimate pulse in the cases that the last pulse has a lower amplitude (see Figure (b)). Calls vary in duration from 102 to 328 ms and are emitted at rates from 7.7 to 23.0 calls/min at irregular intervals. Calls have from 7 to 21 pulses, emitted at rates from 63.5 to 70.4 pulses/s. Pulses steadily increase in amplitude until their final portion, followed by an almost complete amplitude modulation towards the onset of the next pulse. Pulses usually have regular amplitude modulations (3–5 pulse sub-units; Figure ), except for the first pulse, which may be weakly modulated. Pulse duration varies from 12 to 16 ms. Calls have energy distributed along a wide frequency range (mostly from 1500 to 5500 Hz) distributed in a ‘J-shaped’ pattern for each pulse (at 256-point FFT), with the dominant frequency in most cases coincident with the lowest frequencies of the call, from 1593 to 1687 Hz; except from one individual that emitted five out of eleven analyzed calls with the dominant frequency coincident with the highest frequencies, ranging from 2953 to 3046 (3009.4 ± 39.2).
Table 2. Advertisement call traits for six topotypes of S. haddadorum and four topotypes of S. rupestris. Mean ± SD, and n = number of analyzed calls. See Appendix 1 for the analyzed sound files. Asterisk = the dominant frequency of calls of S. haddadorum is contained within a single frequency band (see text).
Figure 3. (a) and (b) Advertisement calls of S. haddadorum from the type locality (Barra do Garças, MT). Spectrogram and corresponding oscillogram of two recorded males: (a) sound file: Scinax_haddadorumBarraGarcasMT1fFSA_AAGb. (b) One advertisement call followed by the second type of note. Sound file: Scinax_haddadorumBarraGarcasMT4aAAGm671.
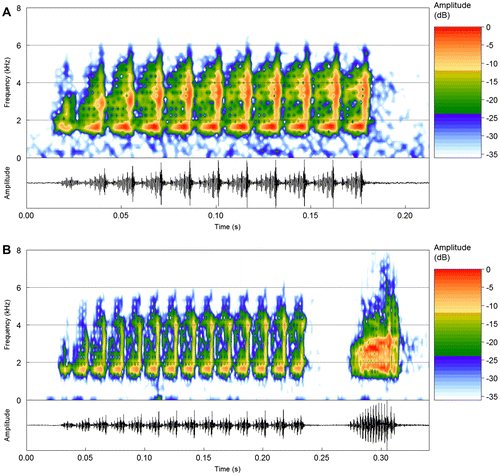
The second note type, tentatively assigned to an aggressive category (sensu Toledo et al. [Citation18]), was emitted shortly after advertisement calls (Figure (b)) or in sequences of up to three notes. Notes have a gradual increase in amplitude until their mid-portion or last third duration, followed by a decay to the last pulse; in some cases, notes can have a longer low amplitude first portion which is sustained until their last third duration, followed by a high amplitude final portion. Aggressive notes last from 32 to 168 ms (55.5 ± 26.5; n = 18) and have from 13 to 38 pulses (20.2 ± 6.2; n = 18), sometimes ill-defined (weak amplitude modulations), especially the first pulses of the notes. The dominant frequency varies from 1640 to 3234 Hz (2780.6 ± 379.7; n = 18).
S. rupestris
Males were found calling on the ground along drainage ditches close (<30 m) to a seasonal, rocky-bottom streamlet bordered by scattered trees. Males were found in calling activity on 28 November 2013.
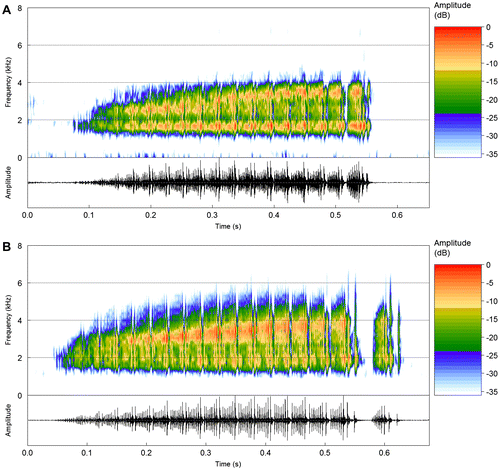
The vocalization (n = 4 recorded males) consists of an advertisement call and two other types of notes. The advertisement call (n = 113 calls; Table , Figure ) is in agreement with Araujo-Vieira et al. [Citation7] and consists of a multipulsed note with a gradual increase in amplitude until its last pulse (call rise time = 94–98%) or until it reaches a sustained plateau approximately in its half or last third (call rise time = 40–70%). Calls vary in duration from 334 to 708 ms and are emitted at irregular intervals. Calls have from 11 to 18 pulses emitted at rates from 22 to 41 pulses/s. Pulses typically have either a progressive increase in amplitude along their duration or a pattern of modulation consisting of pulse onset and offset having higher amplitude compared to a valley around mid-point (which may be displaced towards pulse onset; Figure ). Between-pulse intervals are not well-delimited, except for the last pulse, which may be separated from the others (Figure (b)) by intervals from 5 to 24 ms. The first and last pulses are often remarkably longer than the other pulses. Pulses usually have regular amplitude modulations (7–10 pulse sub-units; Figure (a) and (b)), except for the first pulse, which may be weakly modulated. First pulse duration varies from 42 to 79 ms, last pulse from 39 to 71 ms, and the remaining pulses from 21 to 32 ms. Calls have two emphasized frequency bands. The higher band may shift frequencies along the call (Figure (a)) or broaden the bandwidth of the frequency band (orange-red colors in the spectrogram of Figure (b)), in both cases accompanying the progressive increase in amplitude of the call. The lower frequency band is narrower and has no noticeable frequency shifts (Figure ). The dominant frequency coincides either with the lower (1594 to 2063 Hz) or the higher (3000 to 3876 Hz) frequency band among calls of a same male (except for one male, whose call dominant frequency is coincident with the higher frequency band only).
Figure 4. (a) and (b) Advertisement calls of S. rupestris from the type locality (Alto Paraíso de Goiás, GO). Spectrogram and corresponding oscillogram of two recorded males: (a) sound file: Scinax_rupestrisVeadGO2aCSB_AAGm671. (b) Sound file: Scinax_rupestrisVeadGO3aCSB_AAGm671.
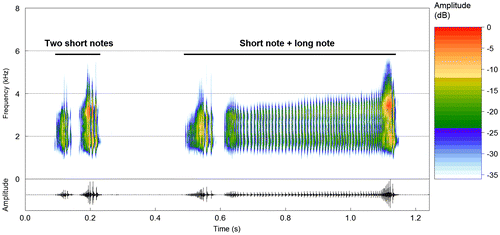
The other two types of notes, tentatively assigned to an aggressive category (Sensu Toledo et al. [Citation18]), were herein referred as ‘short’ and ‘long’ notes. These notes differ from each other by their duration (Figure ). Short notes (n = 115 notes recorded from 4 males) are more commonly emitted than long notes. Short notes can be emitted alone, in sequences of up to four notes, shortly after advertisement calls, or before and/or after long notes. Short notes usually have an increase in amplitude until their mid-point or last third duration, followed by a gradual decay towards the last pulse (Figure ). This note type is very similar to the ‘last pulse’ of advertisement calls since they share a common temporal structure. Short notes last from 32 to 128 ms (65.4 ± 9.9; n = 115) and have from 4 to 30 pulses (12.1 ± 5.1; n = 46), sometimes ill-defined (weak amplitude modulations), especially in their first half. The dominant frequency varies from 1641 to 3422 Hz (2316.6 ± 597.6; n = 115). Long notes (n = 37 notes recorded from 4 males) are almost always emitted shortly after short notes, but may occasionally be emitted alone. Long notes are trills with remarkably lower amplitudes compared to short notes, consisting of a multipulsed structure with low amplitude that is sustained throughout note duration, followed by a high-amplitude final portion, which is similar to a short note (Figure ); also, long notes may have a pattern of gradual increase in amplitude towards the high-amplitude final portion. Long notes last from 291 to 733 ms (513.6 ± 8.8; n = 37) and have from 35 to 95 pulses (60.7 ± 14.8; n = 21), which have short, but evident between-pulse intervals along the low-amplitude portion. The dominant frequency varies from 1641 to 3703 Hz (2815.8 ± 616.1; n = 37).
Figure 5. Other two types of notes of the vocalization of S. rupestris from the type locality (Alto Paraíso de Goiás, GO). Spectrogram and corresponding oscillogram of a sequence of two short notes, followed by a sequence of one short and one long note. Sound File: Scinax_rupestrisVeadGO3aCSB_AAGm671. See Appendix 1 for further information on the sound recordings.
Discussion
Besides the morphological differences, the advertisement call allowed us to establish an acoustic diagnosis for S. haddadorum in relation to the morphologically similar S. rupestris. Call duration of S. haddadorum is relatively short (102–328 ms) compared to that of S. rupestris (334–708 ms; Table ; 290–420 ms [Citation7]). In addition, these species exhibit distinct spectral structure in their calls (under 256-point FFT): the call of S. haddadorum has most energy distributed in a ‘J-shaped’ pattern for each pulse, whereas the call of S. rupestris has two emphasized frequency bands with different bandwidths throughout the call (Table , Figures and ) (see a discussion on frequency bands in calls of other Scinax species in Magrini et al. [Citation13]). Additionally, S. rupestris has a distinct temporal envelope (a progressive increase in amplitude along the call) in relation to that of S. haddadorum (increment in amplitude in the first pulses, followed by a sustained amplitude throughout call duration). Pulse shape is relatively similar in both species by having an upward amplitude modulation (peak amplitude coincident with pulse offset), although pulse duration in S. rupestris (21–32 ms) is longer than it is in S. haddadorum (12–16 ms).
We tentatively assumed that other note types, besides the advertisement ones, were emitted in aggressive contexts (such as males in close-range interactions, spatial maintenance, and others [Citation18,19]). Aggressive notes are often reported for Scinax species [Citation12,20–23], and thus, the assumption that these were emitted in such conditions could be made based on similarities regarding aggressive notes already described for congeners [Citation12,21,23]. It is noteworthy that behavioral experiments are necessary to clearly delimitate the context in which each note is emitted. Additionally, the high variance in the amplitude and emission patterns of the aggressive notes in both species, especially in S. rupestris, might represent a case of graded aggressive notes [Citation24], for which males adjust the attractiveness and aggressiveness of the acoustic output in relation to neighboring males (further discussion in Wells [Citation19]).
Advertisement calls across Scinax species exhibit a great diversity of patterns [Citation13,25]. Thus, additional acoustic traits, such as pulse shape, and patterns of modulation in amplitude and frequency, should be described in future research since we believe that the thorough examination of these acoustic traits might be of great help in the recognition, delimitation, and diagnosis for species of this group of Neotropical treefrogs.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was financially supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais) for AAG lab; a grant by CNPq to AAG; a Masters fellowship by CNPq was conceded to DLB [grant #159817/2015–3]; Doctoral fellowships by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) were conceded to TRC [process # 2012/15763–7] and FSA [process # 2015/10728–7]. Financial support for fieldwork at Parque Estadual da Serra Azul was supported by MCT/CNPq/MMA/MEC/CAPES/FNDCT [grants: Ação Transversal/FAPs #47/2010; CNPq #563134/2010–0].
Authors’ contribution
DLB, TRC and AAG conceived and designed this study and wrote the first drafts of this paper. DLB analyzed calls of S. haddadorum; TRC analyzed calls of S. rupestris. AAG, CSB, FSA, and IAH recorded and collected specimens in the field, reviewed and contributed to the drafts of this study.
Acknowledgements
Fernando Pedroni and Maryland Sanches coordinate biological field studies at Parque Estadual da Serra Azul. Bernardo F. V. Teixeira made available recordings of Scinax rogerioi. Pedro Marinho helped on fieldwork. ICMBio/SISBIO conceded collection permit (#30059–7).
Associate Editor: Juan M. Guayasamin
References
- Faivovich J. A cladistic analysis of Scinax (Anura: Hylidae). Cladistics. 2002;18:367–393.
- Faivovich J, Haddad CFB, Garcia PCA, et al. Systematic review of the frog family Hylidae, with special reference to Hylinae: phylogenetic analysis and taxonomic revision. Bull Am Mus Nat Hist. 2005;294:1–240.
- Duellman WE, Marion AB, Hedges B. Phylogenetics, classification, and biogeography of the treefrogs (Amphibia: Anura: Arboreanae). Zootaxa. 2016;4104(1):1–109.
- Frost, DR. Amphibian species of the world: an online reference (version 6.0). New York (NY): Museum of Natural History. 2016. Available from: http://research.amnh.org/herpetology/amphibia/index.html.
- Andrade FS, Haga IA, Martins FAM, et al. On advertisement call of the poison frog Ameerega berohoka (Dendrobatidae, Anura) from the Brazilian Cerrado. Zootaxa. 2014;3838(3):392–396.
- Araujo-Vieira K, Valdujo PH, Faivovich J. A new species of Scinax Wagler (Anura: Hylidae) from Mato Grosso, Brazil. Zootaxa. 2016;4061(3):261–273.
- Araujo-Vieira K, Brandão RA, Faria DCC. A new species of Rock-Dwelling Scinax Wagler (Anura: Hylidae) from Chapada dos Veadeiros, Central Brazil. Zootaxa. 2015;3915(1):052–066.
- Bioacoustics Research Program. Raven Pro: interactive sound analysis software (version 1.5). Ithaca (NY): The Cornell Lab of Ornithology. 2014. Available from: http://www.birds.cornell.edu/raven.
- Sueur J, Aubin T, Simonis C. Seewave, a free modular tool for sound analysis and synthesis. Bioacoustics. 2008;18:213–226.
- R Development Core Team. R: a language and environment for statistical computing, version 3.2.3. Vienna (AU). 2015. Available from: http://www.R-project.org.
- Littlejohn NJ. Patterns of differentiation in temporal properties of acoustic signals of anurans. In: Ryan MJ, editor. Anuran communication. New York (NY): Oxford University Press; 2001. p. 102–120.
- Magrini L, Giaretta AA. Calls of two Brazilian species of Scinax of the S. ruber clade (Anura: Hylidae). Herpetol notes. 2010;3:121–126.
- Magrini L, Carvalho-e-Silva SP, Bêda AF, et al. Calls of five species of the Scinax ruber (Anura: Hylidae) clade with comments on their taxonomy. Zootaxa. 2011;3066:37–51.
- Nunes I, Kwet A, Pomba JP Jr. Taxonomic revision of the Scinax alter species complex (Anura: Hylidae). Copeia. 2012;3:554–569.
- Martin WF. Mechanics of sound production in toads of the genus Bufo: passive elements. J Exp Zool. 1971;176:273–294.
- Martin WF. Evolution of vocalizations in the genus Bufo. In: Blair WF, editor. Evolution in the genus Bufo. Austin (TX): University of Texas Press; 1972. p. 279–309.
- Gans C. Sound production in the Salientia: mechanism and evolution of the emitter. Amer Zool. 1973;13:1179–1194.
- Toledo LF, Martins IA, Bruschi DP, et al. The anuran calling repertoire in the light of social context. Acta Ethol. 2015;18:87–99.
- Wells KD. The ecology and behavior of amphibians. Chicago (IL): University of Chicago Press; 2007.
- Bastos RP, Haddad CFB. Acoustic and aggressive interactions in Scinax rizibilis (Anura: Hylidae) during the reproductive activity in southeastern Brazil. Amphib-Reptilia. 2002;23:97–104.
- Toledo LF, Haddad CFB. Acoustic repertoire and calling behavior of Scinax fuscomarginatus (Anura: Hylidae). J Herpetol. 2005;39(3):455–464.
- Bastos RP, Alcantara MB, Morais AR, et al. Vocal behaviour and conspecific call response in Scinax centralis. Herpetol J. 2011;21:43–50.
- Carvalho TR, Martins LB, Giaretta AA. The complex vocalization of Scinax cardosoi (Anura: Hylidae), with comments on advertisement calls in the S. ruber Clade. Phyllomedusa. 2015;14(2):127–137.
- Wells KD, Schwartz JJ. Vocal communication in a Neotropical treefrog, Hyla ebraccata: advertisement calls. Anim Behav. 1984;32:405–420.
- De la Riva I, Márquez R, Bösch J. Advertisement calls of Bolivian species of Scinax (Amphibia, Anura, Hylidae). Bijdr Dierkd. 1994;64:75–85.
Appendix 1.
List of the analyzed sound files of S. haddadorum and S. rupestris. Abbreviations of Brazilian states: Minas Gerais = MG; Mato Grosso = MT; Goiás (GO). Information about SVL for S. rogerioi is not available because this specimen is loaned. Temperature for this species recording was not measured.
