Abstract
Extra chromosomes are found in all major taxa, and their characteristics, function, and evolution are not yet completely understood. Known as B chromosomes, they are additional and dispensable genetic material that are present in particular individuals of certain populations of some species. In this work, we present the occurrence of B chromosomes in the cichlid Crenicichla lepidota from southern Brazil. The analyzed population showed 2n = 48 chromosomes, plus 0, 2, 3, or 4 B chromosomes, being variable among metaphases (intraindividual variation). Morphologically, these B chromosomes were variable in size, similarly to the A chromosomes. B chromosomes did not show heterochromatization. Other cytogenetic markers, such as Nucleolar Organizing Region, CMA3/DAPI base-specific dyes, and Fluorescent in situ hybridization with 18S rDNA probe, showed no differences in relation to previous studies with C. lepidota. Although this is the second description of B chromosomes for this species, we highlight the specific features of this population, especially the similarity to A chromosomes, in relation to size, morphology, and heterochromatin accumulation. In summary the B chromosomes described herein are large to small-sized, euchromatic chromosomes, a result that contrasts with that obtained so far for Neotropical cichlids.
Introduction
One way to understand variation among organisms is the study of chromosomal variability. Some species have supernumerary or accessory chromosomes, called B chromosomes. They are additional and dispensable genetic material that are present in particular individuals of certain populations of some species. Although they are apparently dispensable genetic material, B chromosomes can persist in natural populations without being eliminated by natural selection [Citation1]. Possibly, they originated from A chromosomes and follow their own evolutionary pathway [Citation2]. Over the years, researchers have attempted to define B chromosomes, but the characteristics established for them are not always present in all B chromosomes. However, generally, B chromosomes are not homologous with A chromosomes. In addition, they can be morphologically different from A chromosomes, and show a non-Mendelian inherence pattern, mechanisms of accumulation and no nucleolar organizing region (revised in Beukeboom [Citation2]).
B chromosomes have been found in all major animal and plant groups [Citation3], and are estimated to exist in 15% of all living species [Citation2]. There are descriptions of supernumerary chromosomes in fishes, initially in Alburnus alburnus [Citation4] and Prochilodus scrofa (today called P. lineatus) [Citation5]. In fishes, we can find three classes of B chromosomes: (I) large B chromosomes, usually as big as the largest chromosome pair in the karyotype, (II) small B chromosomes, usually the size of the smallest pair of the karyotype, and (III) microchromosomes [Citation6]. The most frequent is the third class, being the occurrence of large B chromosomes infrequent [Citation7].
Looking to the Cichlidae family, there is evidence of B chromosomes, which were described for the first time in germ cells of Gymnogeophagus balzanii [Citation8]. Later, they were observed in Geophagus brasiliensis, Cichlasoma paranaensis, and Crenicichla niederleinii, being described as “corpuscles of chromatin” [Citation9]. Today, B chromosomes have been identified in more than 20 cichlid species (see ). For the South American species, micro or small B chromosomes classes are predominant, while for African species the large-sized B chromosomes are predominant.
Table 1. Occurrence of B chromosomes in Cichlidae. Classes of B chromosomes: (I) large B chromosomes, usually as big as the largest chromosome pair in the karyotype, (II) small B chromosomes, usually the size of the smallest pair of the karyotype, and (III) microchromosomes [Citation6].
Crenicichla lepidota is a South American cichlid distributed in Argentina, Bolivia, Brazil, and Paraguay, along the basins of Paraná River, Paraguay River, Guaporé River, Uruguay River, and along the coast of Rio Grande do Sul (southern Brazilian state) [Citation10]. An invariant diploid number 2n = 48 has been reported for Crenicichla species [Citation11–Citation14], with C. lepidota being no exception [Citation9,15–23]. However, B chromosome polymorphisms have been reported for Amazonian populations of C. reticulata (northern Brazil) [Citation24] and for C. lepidota from Patos Lagoon Basin (southern Brazil) [Citation23]. These populations showed intra- and interindividual variation for presence and number of B chromosomes (zero to three B chromosomes) that are entirely heterochromatic (type III and II, respectively, according to Foresti et al. [Citation6]). In this work, we present evidence of another population of C. lepidota with B chromosomes in the coastal region of southern Brazil, extending the range of extra chromosomes in this species. Differently than the Patos Lagoon population, C. lepidota from Quadros Lagoon shows small to large-sized B chromosomes (type III).
Materials and methods
We analyzed 10 specimens (three females and seven males) of C. lepidota from Quadros Lagoon, southern Brazil. This Lagoon belongs to the Tramandaí River basin and is situated between the cities of Capão da Canoa and Maquiné, in the northwest of Rio Grande do Sul State, Brazil. The samples were taken from the eastern coastal region of Quadros Lagoon (29°45′48.726′′S and 50°3′25.951′′W) ().
Figure 1. (A) Distribution of Crenicichla lepidota [Citation10]. (B) Map showing the localities of C. lepidota with known cytogenetic data: 1 = Miranda [Citation17] and 2 = Comprida Lake, Aquidauana [Citation11] both belonging to the Upper Paraguay River basin (MS, central Western Brazil); 3 = Porto Rico [Citation9], Paraná River Basin (PR, southern Brazil); 4 = Posadas [Citation20], Paraná River (Misiones, northwestern Argentina); 5 = Agronomic Experimental Station of UFRGS and 6 = Capivara Stream [Citation23] both from hydrographic system of the Patos Lagoon (Rio Grande do Sul, southern Brazil); 7 = São Gonçalo Stream and 8 = Polegar Lake [Citation22], southern Patos Lagoon; 9 = Saco da Alemoa, 10 = Gasômetro [Citation23] and 11 = Quadros Lagoon [present study]. Localities 9 to 11 (in red) presented B chromosomes.
![Figure 1. (A) Distribution of Crenicichla lepidota [Citation10]. (B) Map showing the localities of C. lepidota with known cytogenetic data: 1 = Miranda [Citation17] and 2 = Comprida Lake, Aquidauana [Citation11] both belonging to the Upper Paraguay River basin (MS, central Western Brazil); 3 = Porto Rico [Citation9], Paraná River Basin (PR, southern Brazil); 4 = Posadas [Citation20], Paraná River (Misiones, northwestern Argentina); 5 = Agronomic Experimental Station of UFRGS and 6 = Capivara Stream [Citation23] both from hydrographic system of the Patos Lagoon (Rio Grande do Sul, southern Brazil); 7 = São Gonçalo Stream and 8 = Polegar Lake [Citation22], southern Patos Lagoon; 9 = Saco da Alemoa, 10 = Gasômetro [Citation23] and 11 = Quadros Lagoon [present study]. Localities 9 to 11 (in red) presented B chromosomes.](/cms/asset/e41d8959-633f-48a7-a6d3-797834e0e7d4/tneo_a_1429164_f0001_oc.jpg)
Mitotic chromosomes were obtained from the anterior kidney by a conventional air-drying method [Citation25]. Analyses of C-bands were performed to verify the presence of heterochromatic regions in the chromosomes [Citation26]. The impregnation with silver nitrate was applied to identify the NOR (Nucleolar Organizing Region) [Citation27]. The base-specific dyes diamidino-2-phenylindole (DAPI) and Chromomycin A3 (CMA3) were applied to identify the rich region of A-T and G-C base pairs, respectively [Citation28]. The technique of fluorescent in situ hybridization (FISH) [Citation29] was applied with 18S rDNA probe generated by PCR of nuclear DNA from the fish Prochilodus argentus [Citation30]. The probe was labeled with biotin-14-dATP by nick translation according to the manufacturer’s instructions (BioNick Labeling System; Invitrogen/Life Technologies, Carlsbad, CA, USA). A stringency of 77% was maintained in all hybridization mixtures (1 g from each probe, 50% deionized formamide, 10% dextran sulfate, 2 × SSC; pH 7.0–7.2; for 18 h). The detection and amplification of hybridization signals were performed using conjugated streptavidin-FITC (Molecular Probes; Invitrogen).
The chromosomes were analyzed in a Leica® DMLS2 microscope and the fluorescent analyses were performed in a Carl Zeiss® Axiophot epifluorescence microscope, and the chromosome images were captured using the software Case Data Manager Expo 4.0 (Applied Spectral Imaging, Carlsbad, CA, USA). Ninety-four metaphases were analyzed (approximately 10 metaphases by specimen) and the chromosomes were categorized in metacentric/submetacentric (m/sm; two arms) and subtelocentric/acrocentric (st/a; one arm) [Citation31]. Specimen was deposited in the scientific collection at the Federal University of Rio Grande do Sul (identification number: UFGRS11180). The procedures performed with the animals were in accordance with the guidelines of the Ethics Committee for Animal Experimentation from the Federal University of Paraná (number 351), and the sample procedures were authorized by the Instituto Chico Mendes de Conservação da Biodiversidade (SisBio n° 16782-1).
Results
Crenicichla lepidota from Quadros Lagoon showed a diploid number of 48 chromosomes (8 m/sm +40 st/a) plus zero, two, three, or four B chromosomes (). The occurrence of B chromosome was independent of sex, and was variable in each metaphase (intraindividual variation). Seventy percent of the specimens showed supernumerary chromosomes, while 30% had none. Among the former, 20% had two B chromosomes, 40% possessed three B chromosomes, and 20% showed four B chromosomes.
Figure 2. Karyotypes of Crenicichla lepidota from Quadros Lagoon, southern Brazil. (A) 2n = 48. (B) 2n = 48 + 2B. (C) 2n = 48 + 3B, (D) 2n = 48 + 4B. Giemsa stain and C-Band are shown for each karyotype, respectively. Bar represents 5 μm.
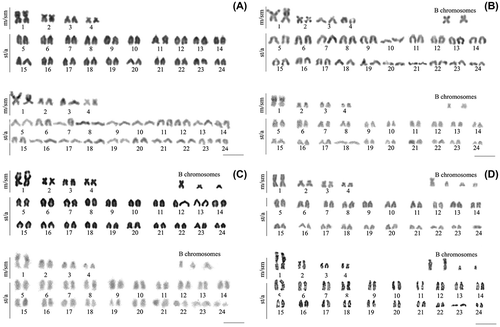
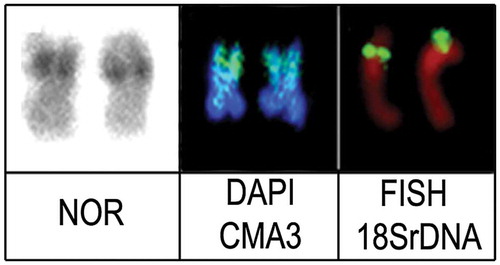
B chromosomes showed similar size and morphology to A chromosomes, with sizes varying from small to large. The mean size of B chromosomes was 1.97 ± 0.7 μm whereas A chromosomes showed a mean of 2.23 ± 0.40 μm. In relation to morphology, the B chromosomes showed metacentric, submetacentric, and subtelo/acrocentric configurations.
Heterochromatin was observed in the secondary constriction of the metacentric first pair, the largest of the complement. Conspicuous blocks of heterochromatin were only recorded on the centromeric regions of the metacentric or submetacentric first pairs. The other chromosomes, including B chromosomes, had only small amounts of heterochromatin or were apparently devoid of it ().
The fluorescent in situ hybridization with 18S rDNA probes resulted in a bright fluorescent signal on a subterminal position of the short arm of metacentric chromosomes (pair 1) that corresponded to the NOR locations evidenced by silver nitrate Ag-NORs, CMA3 positive, and DAPI negative bands in this region ().
Discussion
Despite having very similar characteristics to other Crenicichla populations, such as diploid number, C-bands, and NOR region, C. lepidota from Quadros Lagoon showed some specimens with B chromosomes. This is the second report for B chromosomes in C. lepidota. The first population discovered with extra chromosomes in some individuals was in the Patos-Mirim basin [Citation23]. Despite the proximity of these populations, around 120 km, they show B chromosomes with different characteristics. Pires et al. [Citation23] highlighted the occurrence of small-sized heterochromatic extra chromosomes. This B chromosome size is the most frequent type of karyotype component found in Neotropical Cichlidae [Citation32], which is considered as an additional cytogenetic feature distinguishing the Neotropical from African cichlids. However, Crenicichla cichlids from Quadros Lagoon described in this work seem to be an exception to this rule, because the B chromosomes from this population are as large as the A chromosomes.
In fishes, there are many descriptions of B chromosomes [Citation7,33–40, among others]. It is possible to observe that these chromosomes may vary in relation to size, morphology, and number [Citation41,42]. In cichlids, the B chromosomes were observed as small or punctate chromosomes [Citation8,23,32,33,43–45] or large chromosomes, as in African Haplochromis obliquidens [Citation32]. Oliveira et al. [Citation46] highlighted that B chromosomes from Neotropical fishes are mostly microchromosomes, but there are cases of chromosomes with different sizes and forms, such as in some populations of Astyanax eignmanniorum, A. fasciatus, A. scabripinnis, A. cf. schubarti, Astyanax sp., Apareiodon piracicabae, Oligosarcus pintoi, and Hisonotus leucofrenatus. The present work shows a population of C. lepidota from Quadros Lagoon as one of these cases; in addition, this study also represents an exception among the cichlids bearing supernumerary chromosomes, as this population is the only with B chromosome polymorphisms, including metacentric, submetacentric, subtelocentric, and acrocentric polymorphisms.
Heterochromatization seems to be a common process in the evolutionary history of B chromosomes since these chromosomes are often found totally heterochromatic. However, there are numerous examples of C-banding not occurring or being present only in minor intensity of coloration in these chromosomes. In fishes, this situation has been reported in Moenkhausia sanctaefilomenae [Citation6] Steindachnerina insculpta [Citation47], and Characidium cf. zebra [Citation48]. In Cichlidae, B chromosomes were totally heterochromatic in almost all analyzed species. However, C. lepidota showed differences in relation to heterochromatization of B chromosomes, being heterochromatic in Patos Lagoon population [Citation23] and euchromatic in the Quadros Lagoon population (present work). Venere et al. [Citation48] suggested that the occurrence of euchromatic supernumerary chromosomes in Characiformes species is a strong indication that the origin of these B chromosomes was independent in this fish group; this heterochromatin had no role in the origin and evolution of these chromosomes. Despite the sparse knowledge about B chromosomes in Cichlidae, we can infer that the situation described for Characidae could also occur in Cichlidae, since species with heterochromatic [Citation8,21,23,24,32,43–45,49] and euchromatic B chromosomes (present work) were found. Furthermore, the B chromosomes that differed in heterochromatization, size, and morphology are from two C. lepidota populations within the same region, which has a recent geological formation (about 6.000 years). These differences reinforce the hypothesis of an independent origin of B chromosomes suggested by Venere et al. [Citation48].
The origin of B chromosomes in cichlids has been suggested to be facilitated by the bioaccumulation of heavy metals in C. reticulata and the interbreeding of Cichla monoculus and Cichla sp., both from the Amazon region [Citation24]. For C. lepidota from Quadros Lagoon, the environmental contamination may also be involved in the origin of B chromosomes because this lagoon is embedded in urban centers with environmental problems, as highlighted for the Patos Lagoon population [Citation23]. This situation was observed for most studies with B chromosomes in fishes, which are usually from areas affected by pollution or dam construction. Thus, the hypothesis of supernumerary chromosome origin as a spinoff of environmental stress is plausible for C. lepidota, leading to abnormal chromosomes, which then remain in populations through chromosomal polymorphism [Citation46]. In this hypothesis, non-disjunction meiotic errors followed by B chromosome accumulation mechanisms due to non-Mendelian transmission may explain the high population frequency of these chromosomes [Citation50].
In summary, this work showed the polymorphic characteristic of B chromosomes in C. lepidota from Quadros Lagoon, Tramandaí River basin, which are similar to A chromosomes in relation to size, morphology, and heterochromatin accumulation. This is the second description of B chromosomes for this species; however, but we highlight the specific features of this population. Their B chromosomes are small to large-sized chromosomes, a divergent result regarding what was found so far for most Neotropical cichlids. C. lepidota from Tramandaí River basin and Patos Lagoon basin seem to follow a different evolutionary pathway in their B chromosomes, corroborating with the hypothesis of an independent origin of B chromosomes. Nowadays, questions regarding the molecular content, origin, and evolution of B chromosomes are being addressed by high-throughput sequencing [Citation33]. We highlight the importance of future work on the study of meiosis and synaptonemal complex using other techniques, such microdissection and molecular approaches to understand the importance of these B chromosomes in populations of C. lepidota.
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS/CNPq-PRONEX, number 16/0485-4); Fundação Araucária de Apoio ao Desenvolvimento Científico e Tecnológico do Estado do Paraná.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
The authors are grateful to the team of Professor Luiz Malabarba from Universidade Federal do Rio Grande do Sul for identifying and cataloging specimens.
Associate Editor: Federico Brown.
References
- Carvalho RA, Martins-Santos IC, Dias AL. B chromosomes: an update about their occurrence in freshwater Neotropical fishes (Teleostei). J Fish Biol. 2008;72(8):1907–1932. DOI:10.1111/j.1095-8649.2008.01835.x
- Beukeboom LW. Bewildering Bs: an impression of the 1st B-chromosome conference. Heredity (Edinb). 1994;73(March):328–336.10.1038/hdy.1994.140
- Camacho JPM. B chromosomes in the Eukaryote genome – preface. Cytogenet Genome Res. 2004;106:147–148.10.1159/000080118
- Hafez R, Labat R, Quillier R. Recherches sur les chromosomes surnuméraires de L’ablette (Alburnus alburnus) [Research on supernumerary chromosomes. The bleak (Alburnus alburnus)]. Cybium. 1981;5:81–87.
- Pauls E, Bertollo LAC. Distribution of a suprernumerary chromosome system and aspects of karyotypic evolution in the genus Prochilodus (Pisces, Prochilodontidae). Genetica. 1983;81:117–123.
- Foresti F, Almeida-Tolcdo LF, Toledo SA. Supernumerary chromosome system, C-banding pattern characterization and multiple nucleolus organizer regions in Moenkhausia sanctaefilomenae (Pisces, Characidae). Genetica. 1989;79(2):107–114. DOI:10.1007/BF00057927
- Maistro EL, Oliveira C, Foresti F. Cytogenetic analysis of A- and B-chromosomes of Prochilodus lineatus (Teleostei, Prochilodontidae) using different restriction enzyme banding and staining methods. Genetica. 2000;108(2):119–125. DOI:10.1023/A:1004063031965
- Feldberg E, Bertollo LAC. Discordance in chromosome number among somatic and gonadal tissue cells of Gymnogeophagus balzanii (Pisces: Cichlidae). Rev Bras Genet. 1984;4(VII):639–645.
- Martins IC, Portela-Castro ALDB, Julio Junior HF. Chromosome analysis of 5 species of the Cichlidae family from the Parana River. Cytologia (Tokyo). 1971;1995(60):223–231.
- Reis R, Lima F. Crenicichla lepidota. The IUCN red list of threatened species. T167717A6372093.
- Loureiro MA, Caetano LG, Dias AL. Cytogenetic characterization of two Species Crenicichla (Pisces, Cichlidae) of the genus. Cytologia (Tokyo). 2000;65:57–63.10.1508/cytologia.65.57
- Valente GT, Vitorino CDA. Cytogenetics comparative cytogenetics of ten species of cichlid fishes (Teleostei, Cichlidae) from the Araguaia River system, Brazil, by conventional cytogenetic methods. Comp Cytogen. 2012;6(2):163–181. DOI:10.3897/CompCytogen.v6i2.1739
- Molina WF, Pacheco GA, Miron W, et al. Padrões citogenéticos de duas espécies de ciclídeos de bacias do semi-árido do Brasil: Crenicichla menezesi e Cichlasoma Orientale. Biota Amaz. 2014;4(4):33–39.10.18561/2179-5746/biotaamazonia
- Benzaquem DC, Feldberg E, Ivan J, et al. Cytotaxonomy and karyoevolution of the genus Crenicichla (Perciformes, Cichlidae). Genet Mol Biol. 2008;31(1):250–255.10.1590/S1415-47572008000200016
- Schell JJ. Fish chromosomes and their evolution. Internal report of Danmarks Akvarium. Charlottenlund, Denmark; 1973. p. 1–22.
- Thompson KW. Cytotaxonomy of 41 species of neotropical Cichlidae. Copeia. 1979;1979(4):691.
- Feldberg E, Bertollo LAC. Karyotypes od 10 species of Neotropical cichlids (Pisces, Perciformes). Caryologia. 1985;38:257–268.
- Feldberg E, Rebelo-Porto JI, Bertollo LAC. Chromosomal changes and adaptation of cichlid fishes during evolution. In: Val AL, Kapoor BG, editors. Fish Adaptation. Enfield (NH): Science Publishers; 2003. p. 285–308.
- Fenocchio AS, Pastori MC, Roncati HA, et al. A cytogenetic survey of the fish fauna from Argentina. Caryologia. 2003;56(2):197–204.10.1080/00087114.2003.10589325
- Roncati HA, Pastori MC, Fenocchio AS. Fishes of the Family Cichlidae (Perciformes) from Parana River (Argentina). Cytologia (Tokyo). 2007;72(4):379–384.10.1508/cytologia.72.379
- Poletto AB, Ferreira IA, Cabral-de-Mello DC, et al. Chromosome differentiation patterns during cichlid fish evolution. BMC Genet. 2010;11(1):50. DOI:10.1186/1471-2156-13-2
- Perazzo G, Noleto RB, Vicari MR, et al. Chromosomal studies in Crenicichla lepidota and Australoheros facetus (Cichlidae, Perciformes) from extreme Southern Brazil. Rev Fish Biol Fish. 2010;21:509–515. DOI:10.1007/s11160-010-9170-x
- Pires LB, Sampaio TR, Dias AL. Mitotic and meiotic behavior of B chromosomes in Crenicichla lepidota: new report in the family Cichlidae. J Hered. 2015;106(3):289–295. DOI:10.1093/jhered/esv007
- Feldberg E, Porto JIR, Alves-Brinn MN, et al. B chromosomes in Amazonian cichlid species. Cytogenet Genome Res. 2004;106:195–198. DOI:10.1159/000079287
- Bertollo LA, Takahashi CS, Moreira-Filho O. Cytotaxonomy considerations on Hoplias lacerdae (Pisces, Erythrinidae). Brazilian J Genet. 1978;1:103–120.
- Sumner AT. A simple technique for demonstratig centromeric heterochromatin. Exptl Cell Res. 1972;75:304–306.10.1016/0014-4827(72)90558-7
- Howell WM, Black DA. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia. 1980;36:1014–1015.10.1007/BF01953855
- Schweizer D. Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma. 1976;58:307–324.10.1007/BF00292840
- Pinkel D, Straume T, Gray JW. Cytogenetic analysis using quantitative, high sensitivity, flourescent hybridization. Proc Natl Acad Sci USA. 1996;83:2234–2938.
- Hatanaka T, Galetti PM. Mapping of the 18S and 5S ribosomal RNA genes in the fish Prochilodus argenteus Agassiz, 1829 (Characiformes, Prochilodontidae). Genetica. 2004;122(3):239–244.10.1007/s10709-004-2039-y
- Levan A, Fredga K, Sandberg AA. Nomenclature for centromeric position at chromosomes. Hereditas. 1964;201–220. DOI:10.1111/j.1601-5223.1964.tb01953.x
- Poletto AB, Ferreira IA, Martins C. The B chromosomes of the African cichlid fish Haplochromis obliquidens harbour 18S rRNA gene copies. BMC Genet. 2010;11:1. DOI:10.1186/1471-2156-11-1
- Valente GT, Conte MA, Fantinatti BEA, et al. Origin and evolution of B chromosomes in the cichlid fish Astatotilapia latifasciata based on integrated genomic analyses. Mol Biol Evol. 2014;31(8):2061–2072. DOI:10.1093/molbev/msu148
- Maistro EL, Foresti F, Oliveira C, et al. Occurrence of macro B chromosomes in Astyanax scabripinnis paranae (Pisces, Characiformes, Characidae). Genetica. 1992;87(2):101–106. DOI:10.1007/BF00120999
- Maistro EL, Oliveira C, Foresti F. Cytogenetic characterization of a supernumerary chromosome segment and of B-chromosomes in Astyanax scabripinnis (Teleostei, Characidae). Genetica. 2001;110(2):177–183. DOI:10.1023/A:1017961411614
- Néo DM, Bertollo LC, Filho OM. Morphological differentiation and possible origin of B chromosomes in natural Brazilian population of Astyanax scabripinnis (Pisces, Characidae). Genetica. 2000;108(3):211-215. DOI:10.1023/A:1004157901097
- Néo DM, Filho OM, Camacho JPM. Altitudinal variation for B chromosome frequency in the characid fish Astyanax scabripinnis. Heredity. 2000;85(2):136–141. DOI:10.1046/j.1365-2540.2000.00744.x
- Portela-Castro ALDB, Júlio-Junior HF, Nishiyama PB. New occurrence of microchromosomes B in Moenkhausia sanctaefilomenae (Pisces, Characidae) from the Paraná River of Brazil: Analysis of the synaptonemal complex. Genetica. 2001;110:277–283. DOI:10.1023/A:1012742717240
- Noleto RB, Vicari MR, Cestari MM, et al. Variable B chromosomes frequencies between males and females of two species of pufferfishes (Tetraodontiformes). Rev Fish Biol Fish. 2011;22(1):343–349. DOI:10.1007/s11160-011-9231-9
- Silva DMZA, Pansonato-Alves JC, Utsunomia R, et al. Delimiting the origin of a B chromosome by FISH mapping, chromosome painting and DNA sequence analysis in Astyanax paranae (Teleostei, Characiformes). PLoS One. 2014;9(4):33–36. DOI:10.1371/journal.pone.0094896
- Salvador LB, Moreira-Filho O. B chromosomes in Astyanax scabripinnis (Pisces, Characidae). Heredity. 1992;69(1):50–56.10.1038/hdy.1992.93
- Artoni RF, Shibatta OA, Gross MC, et al. Astyanax aff. fasciatus Cuvier, 1819 (Teleostei; Characidae): evidences of a species complex in the upper rio Tibagi basin (Paraná, Brazil). Neotrop Ichthyol. 2006;4(2):197–202. DOI:10.1590/S1679-62252006000200005
- Fantinatti BEA, Mazzuchelli J, Valente GT, Cabral-de-Mello DC, Martins C. Genomic content and new insights on the origin of the B chromosome of the cichlid fish Astatotilapia latifasciata. Genetica. 2011;139(10):1273–1282. DOI:10.1007/s10709-012-9629-x
- Yoshida K, Terai Y, Mizoiri S, et al. B chromosomes have a functional effect on female sex determination in lake victoria cichlid fishes. PLoS Genet. 2011;7(8):e1002203. DOI:10.1371/journal.pgen.1002203
- Kuroiwa A, Terai Y, Kobayashi N, et al. Construction of chromosome markers from the lake victoria cichlid Paralabidochromis chilotes and their application to comparative mapping. Cytogenet Genome Res. 2014;142(2):112–120. DOI:10.1159/000356128
- Oliveira C, Foresti F, Hilsdorf S. Genetics of neotropical fish: from chromosomes to populations. Fish Physiol Biochem. 2009;35:81. DOI:10.1007/s10695-008-9250-1
- Oliveira C, Foresti F. Occurrence of supernumerary microchromosomes in Steindachnerina insculpta (Pisces, Characiformes, Curumatidae). Cytobios. 1993;76:183–186.
- Venere PC, Miyazawa CS, Galetti PM. New cases of supernumerary chromosomes in characiform fishes. Genet Mol Biol. 1999;22(3):345–349. DOI:10.1590/S1415-47571999000300010
- Valente GT, Schneider CH, Gross MC, et al. Comparative cytogenetics of cichlid fishes through genomic in situ hybridization (GISH) with emphasis on Oreochromis niloticus. Chromosom Res. 2009;17(6):791–799. DOI:10.1007/s10577-009-9067-5
- Camacho JPM, Sharbel TF, Beukeboom LW. B-chromosome evolution. Philos Trans R Soc B Biol Sci. 2000;355(1394):163–178. DOI:10.1098/rstb.2000.0556