ABSTRACT
Green bottle flies (Diptera, Calliphoridae, Luciliinae) comprise a diverse and cosmopolitan taxon, known from at least 1,500 species. They have become crucial elements in forensic investigations, as they spend part of their life cycle in decaying remains. Here, we review the distribution of eleven Luciliinae species in Ecuador: the monotypic Blepharicnema and ten Lucilia species. We identified specimens using morphological characters. Additionally, we DNA barcoded 43 specimens from three species using 658bp segments of the standard Cytochrome Oxidase I (COI) mitochondrial gen. Molecular and morphological identifications presented high correspondence, suggesting COI barcodes are an efficient tool for the identification of these three green bottle flies species. Geographical records are biased towards the northern Andean region, particularly near to large urban settlements. We remark the value to applied forensic research of continuous sampling of necrophagous flies under a variety of habitats and crime conditions.
Introduction
Approximately 160,000 species of Diptera have been described worldwide [Citation1,Citation2]. Flies play fundamental ecological functions, like pollination, biological control, decomposition of organic material [Citation2–4], among others. Blow flies (Diptera: Calliphoridae) comprise over 1,500 species and are considered a major group of insects in the process of carcass animal reduction [Citation5–10]. Neotropical Calliphoridae encompasses four subfamilies: Calliphorinae, Chrysomyinae, Toxotarsinae, and Luciliinae [Citation11,Citation12]. Approximately 100 species of blow flies are found in the Neotropics [Citation10]. To date, two genera of Luciliinae, species of which are commonly known as green bottle flies, had been reported in the Neotropics: Lucilia (23 spp.) and the monotypic Blepharicnema [Citation11,Citation12]. Besides their role in animal decomposition, green bottle flies are efficient mechanical vectors of diseases like myiasis (tissue infestation with fly larvae) [Citation13], while other species are widely used in treatments of larval therapy, bio-therapy and bio-surgery [Citation14]. However, our understanding of the diversity and distribution of green bottle flies in Ecuador remains factionary [Citation6,Citation11,Citation12].
Forensic entomology provides an emerging tool that combines biological and ecological knowledge of carrion species [Citation4,Citation7–9,Citation15]. For example, the time since colonization by necrophagous flies of dead bodies is used to interpret forensic evidence in criminal cases [Citation7–9]. Thus, correct identification of target fly specimens is an essential early step [Citation15–17] in legal investigations. However, little research has been done on forensically important flies in Ecuador. Here, we aim to assess the diversity of Luciliinae species associated with carrion by presenting an updated checklist and distribution maps along continental and insular Ecuador. Additionally, we provide DNA COI barcodes for three of these species. Our broader aim is to contribute with geographical data of taxonomically confirmed voucher specimens to the practical use of forensic entomology and faunal inventories in Ecuador.
Materials and methods
Checklist
We include in this checklist specimen information from field trip collections, museum specimens, and a review of literature. Field trips were done from 2013 to 2016, covering disturbed and natural habitats at eight sites (ranging from 200 to 2800 m asl) throughout the Andean highlands and the Amazon region of Ecuador. We used modified McPhail, Van Someren-Rydon, and pitfall traps with decaying baits (fish heads, shrimps, meat, chicken viscera), as well as a pig (Sus scrofa) and guinea pig (Cavia porcellus) carcasses, to attract important forensic arthropods. In the laboratory, we sorted and identified approximately 3200 specimens and reared larvae to mature stages when possible. We dissected genitalia for both sexes to verify the identity of those species represented by ten individuals or more (males and females when possible). We included 114 voucher specimens deposited at the Invertebrate Section of the QCAZ Museum, in the Pontificia Universidad Católica del Ecuador, with collection dates ranging from 1992 to 2016. Finally, we extracted from the scientific literature occurrence records of Luciliinae flies for Ecuador. We created distribution maps using georeferenced localities in wgs84 geodetic datum for each Luciliinae species with Quantum GIS (QGIS) 2.2. Taxonomic identification follows the keys of Whitworth [Citation11].
Museum abbreviations stand for:
QCAZ Quito Católica Zoología. Sección Invertebrados, Quito. Ecuador.
MECN Museo Ecuatoriano de Ciencias Naturales, Quito, Ecuador.
CNC Canadian National Collection of Insects, Agriculture and Agri-Food Canada, Canada.
LACM Los Angeles County Museum of Natural History, Los Angeles, USA.
UGG University of Guelph, Guelph, Canada.
WSUP M.T. James Entomological Collection, Washington State University, Pullman, USA.
DNA COI barcoding
To aid in species identification, we barcoded 43 specimens previously identified with morphological characters as Lucilia purpurascens, L. sericata and L. eximia. We amplified and sequenced samples in collaboration with Canadian Centre for DNA Barcoding (CCDB) following the standard protocols of the Biodiversity Institute of Ontario, Guelph University [Citation18], using C_LepFolF and C_LepFolR primers. Sequences are available in GenBank under the accession numbers MF458318 to MF458358, and in the BOLD (www.boldsystems.org/) database under the public project FFECU – Forensic Flies of Ecuador. After general verification of congruence between morphological and molecular taxonomic units, each species was given a Barcode Index Number (BIN) by BOLD. BINs can be used as a proxy taxonomic unit [Citation17] and provides a permanent and objective molecular reference for further studies.
Results
Checklist
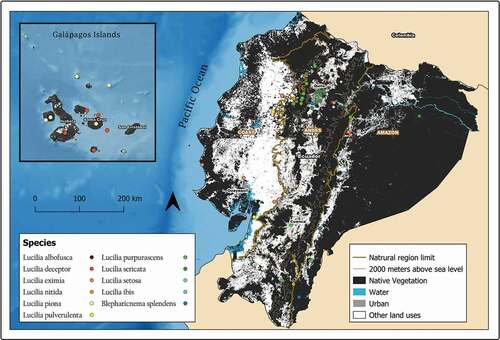
Examined specimens belong to eleven Luciliinae species () occurring along 53 localities from 14 provinces in continental and insular Ecuador (). Visual inspection of distribution maps showed the northern Andean highlands as the most sampled area within the country.
Figure 3. Map showing localities for the distribution of Lucilia albofusca, L. eximia, and L. pulverulenta in continental Ecuador
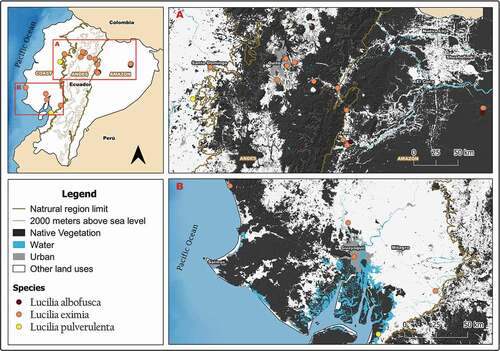
Figure 4. Map showing localities for the distribution of Lucilia purpurascens and L. nitida in continental Ecuador
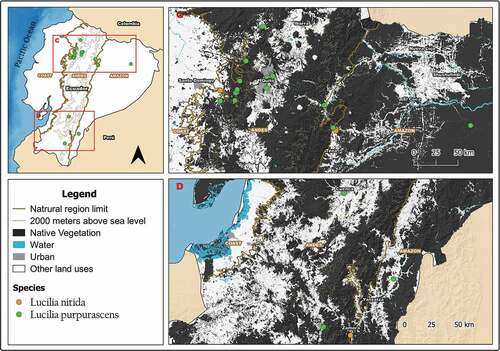
Figure 5. Map showing localities for the distribution of Lucilia ibis, L. sericata and Blepharicnema splendens in continental Ecuador
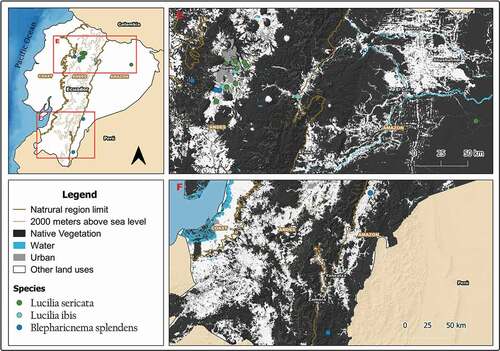
Figure 6. Map showing localities for the distribution of Lucilia deceptor, L. piona and L. setosa in the Galapagos Archipelago, Ecuador
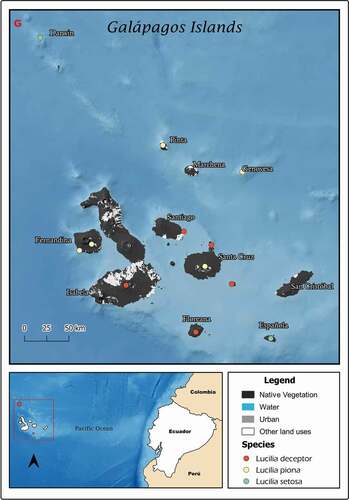
Table 1. Checklist of green bottle flies (Calliphoridae: Luciliinae) of Ecuador. Abbreviations; PAC: Pacific coast; AND: Andean region; AMA: Amazonia; GAL: Galapagos Insular region. Imb: Imbabura; Mor: Morona Santiago; Zam: Zamora; Orell: Orellana; Pichin: Pichincha; Esp: Española; Isab: Isabela; Barto: Bartolome; Flore: Floreana; Sey: Seymour; Sto. Dom: Santo Domingo; Guay: Guayas; Sta. Ele: Santa Elena; Fernan: Fernandina; Geno: Genovesa; Coto: Cotopaxi; Azu: Azuay
DNA COI barcoding
We obtained 43 complete COI sequences from three Lucilia species from Ecuador. Molecular clustering of these 43 specimens within previously barcoded species for the country (sequences available in Genbank) showed high taxonomic concordance (). Molecular clustering of these 43 specimens within a total of 2416 public sequences from 62 putative species is presented in Appendix 1. A BIN analyses of the World dataset suggest that our putative species name for the L. sericata and L. eximia fall well within the BINs associated to these species elsewhere in the World (). L. sericata BIN AAA6618 was widespread, and found in at least 14 other countries, including Australia, Canada, China, South Africa, Lebanon and the USA. Across the world, L. eximia showed high molecular variability clustered in at least five BINs; with the Ecuadorian BIN AAD0711 spread across Brazil, Costa Rica, Venezuela and the USA. Our Lucilia purpurascens Ecuadorian specimens clustered in two BIN (AAV7412 and ACS3321); however, none of them clustered with BIN ACF4608 from Costa Rica.
Figure 7. Evolutionary analysis by Maximum Likelihood method of Lucilia barcodes in Ecuador. The evolutionary history was inferred by using the Maximum Likelihood method and Kimura 2-parameter model [Citation43]. The tree with the highest log likelihood (−1517.85) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 0.4209)). The rate variation model allowed for some sites to be evolutionarily invariable ([+I], 76.10% sites). The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. This analysis involved 55 nucleotide sequences. Codon positions included were 1st+2nd+3rd+Noncoding. There were a total of 654 positions in the final dataset. Evolutionary analyses were conducted in MEGA X (Citation44–45)
![Figure 7. Evolutionary analysis by Maximum Likelihood method of Lucilia barcodes in Ecuador. The evolutionary history was inferred by using the Maximum Likelihood method and Kimura 2-parameter model [Citation43]. The tree with the highest log likelihood (−1517.85) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 0.4209)). The rate variation model allowed for some sites to be evolutionarily invariable ([+I], 76.10% sites). The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. This analysis involved 55 nucleotide sequences. Codon positions included were 1st+2nd+3rd+Noncoding. There were a total of 654 positions in the final dataset. Evolutionary analyses were conducted in MEGA X (Citation44–45)](/cms/asset/9faae65d-faf4-41a9-a895-89e22e86b400/tneo_a_1804747_f0007_b.gif)
Table 2. Barcode index numbers (BINs) associated to Ecuadorian Lucilia, and their distribution across the world
Species List
Subfamily Luciliinae
Genus Blepharicnema Macquart, 1843
Blepharicnema splendens Macquart, 1843 ()
Locality records
Ecuador (five females) Imbabura, Los Cedros, 1880 m asl, 0.331 S, 78.781 W, 15/8/2006, R. Cárdenas, QCAZ 224621–24, QCAZ 12065, (QCAZ); (one male), Zamora, Podocarpus, Romerillos, 2200 m asl, 0.415 LS, 78.553 LW, 31/8/1998, S. Noriega, MECN12313 (MECN); (one female) Zamora, Podocarpus, La Curintza, 1787, 0.409 LS, 78.582 LW, 3/9/1998, G. Estevez MECN 12194 (MECN); (one female), Morona Santiago, Tinajillas, −2,96667 − 78,4167, 4/3/1985, F. Brand MECN12246 (MECN); (one female) Zamora, Podocarpus, Sendero Nangaritza, 4.368 LS, 78.835 LW, 3/9/1998, G Estevez MECN12198 (MECN).
Comments
This species is possibly the largest blow fly in the Neotropics [Citation19]. Although most of the natural history of this fly is still unknown, previous studies show that they are strongly attracted to fish bait [Citation19]. Reported for the first time in the Ecuadorian Andes from a specimen collected at Pichincha, Quito by James (1970). Also, B. splendens was reported for Ecuador by [Citation10,Citation19–21]. The material examined from the QCAZ Museum belongs to Imbabura, Zamora and Morona Santiago Provinces.
Distribution
B. splendens is a Neotropical species endemic to the Andes of Peru, Colombia, Bolivia, Ecuador and Venezuela [Citation19].
Genus Lucilia (Robineau-Desvoidy, 1830)
Lucilia albofusca Whitworth, 2014 ()
Locality records
Ecuador (two females) Orellana, EC Yasuni, 250 m asl, 0.671 LS, 76.403 LW, 30/10/2002, C. Bramme (LACM); (one female), Napo, Misahualli, 1.035 LS, 77.776 LW, 26/7/2000, Steven and Paul Keller (LACM); (two females), Napo, Rio Saragaco, 29/1/1997, J. Skartveit (RC); (one female), Orellana, EC Yasuni, 250 m asl, 0.671 LS, 76.403 LW, 28/5/2009, L. Hewitt (UG), (one female), Napo, Coca, Napo River, 12/04 – 30/05/1965, 250 m asl, L. Pena (CNC).
Comments
Reported for the first time in Ecuador by Whitworth [Citation11] based on specimens collected in Napo and Orellana Provinces. Paratypes are deposited in the LAMC, CNC and UGG Museums. Males are very difficult to collect considering only 3% of specimens obtained from carrion bait traps in French Guiana were L. albofusca in [Citation11].
Distribution
L. albofusca is mainly present in the tropical region, where it has been reported in Brazil, Colombia, Ecuador, French Guiana, Guyana, Panama, Peru, Suriname and Venezuela [Citation11].
Lucilia deceptor (Curran, 1934) ()
Locality records
Ecuador (one female, one male), Galapagos, Isla Fernandina, 26/1/1899 (WSUP); (two males) Galapagos, Isla Española, 18/5/1899 (WSUP); (one female) Galapagos, Isla Española, Punta Juarez, 10/12/1967, Ira L. Wiggins (WSUP) [Citation22]; mentioned (one male) from Galapagos, Isla Isabela; (one male) Galapagos, Isla Bartolomeo; Galapagos, Isla Floreana and Galapagos, Isla Seymour Norte.
Comments
First record reported in Isla Española, Galapagos in 1899. Reported for Ecuador by [Citation10,Citation23,Citation24]. No specimens of this species were collected or reviewed.
Distribution
Endemic to the Galapagos Archipelago [Citation10,Citation11,Citation22].
Lucilia eximia (Wiedemann, 1819) ()
Locality records
Ecuador (two males, one female), Orellana, EC Yasuni, 194 m asl, 0.671 LS, 76.402 LW, 3/3/2014, P. Padilla, QCAZ 121821 [MF458331], QCAZ 121820 [MF458337], QCAZ 121808 [MF458324], (QCAZ); (two females, four males) Orellana, EC Yasuni, 195 m asl, 0.671 LS, 76.402 LW, 1/3/2014, M. Castro, QCAZ 121823 [MF458325], QCAZ 121824 [MF458335], QCAZ 121825 [MF458338], QCAZ 121830 [MF458333], QCAZ 121831 [MF458336] (QCAZ); (one female) Orellana, EC Yasuni, 201 m asl, 0.671 LS, 76.402 LW, 3/3/2014, D. Nieto, QCAZ 115003 [MF458328], (QCAZ); (ten females, two males), Pichincha, Puembo, 2592 m asl, 0.202 LS, 78.328 LW, 29/7/2016, A. Torres, QCAZ 212384–86, QCAZ 212388, QCAZ 212390–95, QCAZ 212398, QCAZ 212359, (QCAZ); (two females, one male) Pichincha, Amaguaña, 2618 m asl, 0.385 LS, 78.489 LW, 29/7/2016, A. Torres, QCAZ 212389, QCAZ 212360, QCAZ 212361, (QCAZ); (two females), Pichincha, Cerro Ilalo, 2728 m asl, 0.239 LS, 78.406 LW, 29/7/2016, A. Torres, QCAZ 212396 (QCAZ); (one female), Pichincha, Santo Domingo, 0.254 LS, 79.186 LW, 8/5/1988, M. Bohart; (one male), Pichincha, Nayon, 2397 m asl, 0.166 LS, 78.416 LW, 18/10/2013, S. Aguirre; (one male, two females) Napo, Tena, 561 m asl, 0.998 LS, 77.836 LW, 30/10/2015, M. Dominguez, QCAZ 212268 [MF458326], QCAZ 212272 [MF458323], QCAZ 212266 [MF458329], (QCAZ); (one male, one female) Napo, Sarayacu, 1312 m asl, 0.695 LS, 77.800 LW, 30/10/2015, M. Dominguez, QCAZ 212267 [MF458332], QCAZ 202271 [MF458330] (QCAZ); (one female) Napo, Baeza, 1949 m asl, 0.467 LS, 77.892 LW, 1/11/2015, M. Dominguez, QCAZ 212269 [MF458327], (QCAZ); (one male); Napo, Misahualli, 1.035 LS, 77.776 LW, 25/6/1976, P.M. Turner; (three males), Imbabura, Taguando, 0.400 LS, 78.133 LW, 9/6/1965, L. Pena; (one female), Cañar, El Valle de Cochancay, 280 m asl, 2.421 LS, 79.342 LW, 13/2/1966, R.H. Arnett, E.J. Gerberg; (one male), Guayas, Balao Chico, LS 2.733, 79.750 LW, 23/4/1963, L.E. Pena; (one female), Guayas, El Triunfo, 1.933 LS, 79.966 LW, 4/3/1965, L. Pena; (one male) Guayas, Guayaquil, 2.176 LS, 79.923 LW, 1935, G. Von Buchwald; (one female), Santa Elena, Rio Ayampe, 1.669 LS, 80.809 LW, 26/7/1976, J. Cohen. (one female), Pichincha, Otongachi, 0.383 LS, 78.966 LW, 22/3/2014, G. Rivadeneira, QCAZ 115004 [MF458334].
Comments
First record in Galapagos Islands (Floreana, Santa Cruz and San Cristobal) in 1989, det. B.J. Sinclair. Reported by Salazar & Donoso, and Aguirre [Citation21,Citation22] from QCAZ samples collected in Nayon, 2013 and by [Citation10,Citation11,Citation22,Citation23,Citation25] based on material from Imbabura, Pichincha, Napo, Guayas, Santa Elena and Cañar collected during 1935 to 1988 and housed on the CNC Museum collection. The material examined from the QCAZ Museum was collected in Orellana, Napo and Pichincha Provinces. Individuals of L. eximia can develop on rotten vegetables and fruits, and are known as a secondary myiasis producer [Citation28]. According to COI data in previous phylogenetic studies, L. eximia might be a species complex [Citation11].
Distribution
L. eximia is a common species in the New World and it is commonly found in every country from the southern United States to southern South America, including Argentina, Belize, Bolivia, Brazil, Colombia, Costa Rica, Ecuador, El Salvador, French Guiana, Honduras, Guatemala, Guyana, Mexico, Nicaragua, Panama, Paraguay, Peru, Suriname, Uruguay, Venezuela, and the West Indies [Citation11].
Lucilia ibis Shannon, 1926 ()
Locality records
Ecuador (one male), Pichincha, Quito, 2851 m asl, 0.190 LS, 78.500 LW, 16/7/2016, A. Torres, QCAZ 224464 (QCAZ); (three females), Pichincha, Puembo, 2594 m asl, 0.202 LS, 78.328 LW, 29/7/2016, A. Torres, QCAZ 224466–68; 2554 m asl, (QCAZ); (one female), Pichincha, Cerro Ilalo, 2728 m asl, 0.239 LS, 78.406 LW, 29/7/2016, A. Torres, QCAZ 224469, (QCAZ); (one female), Pichincha, Amaguaña, 2618 m asl, 0.385 LS, 78.489 LW, 29/7/2016, A. Torres, QCAZ 224472, (QCAZ); (five females, two males), Napo, Baeza, 1949 m asl, 0.467 LS, 77.892 LW, 1/11/2015, M. Dominguez, QCAZ 224474–77, QCAZ 224479, (QCAZ); (one female), Pichincha, Nayon, 0.166 LS, 78.416 LW, 18/10/2013, S. Aguirre.
Comments
Reported for the first time in Ecuador by Whitworth [Citation11] based on material housed on the CNC Museum collected at Nayon in 2013. The material examined from the QCAZ Museum belonged to Pichincha and Napo Provinces. It is a relatively common Andean fly, attracted by cow and dog feces and by fish and liver bait. Their role as pollinators is suggested due to their common visitation frequency to flowers [Citation26].
Distribution
The distribution of L. ibis is only known from the Andes highlands in Peru, Bolivia, Ecuador and Argentina [Citation11]. In Ecuador this species is reported in the Andean and Amazonian region from 1900–2900 m asl (this study).
Lucilia nitida Whitworth, 2014 ()
Locality records
Ecuador (one female), Napo, Narupa, 1240 m asl 0.727 LS, 77.772 LW, 07/05/2015, E. Amat, TdeA-7040; (one male) Pichincha, Otongachi, 889 m asl, 0.314 LS, 78.950 LW, S. Aguirre, 7/10/2014 QCAZ L194: (one female), Loja, P. N. Podocarpus, 1027 m asl, 4.102 LS, 78.948 LW, 20/6/2016, E. Moreno, TdeA10073.
Comments
Reported in Ecuador for the first time in 2014 in Napo province during this study.
Locality records
Ecuador (three females), Galapagos, Isla Pinta, Ibbetson, 0.953 LS, 90.965 LW, 13/3/1992, S. Peck; (two females), Galapagos, Isla Fernandina, Cabo Hammond, 0.953 LS, 90.965 LW, 4/5/1991, S. Peck; (one female), Galapagos, Isla Española, Bahia Manzanillo, 0.953 LS, 90.965 LW, 8/6/1985, S. Peck; Tatwain and Sinclair, 2013 mentioned (one female) Galapagos, Fernandina, (one male, 1 female), Galapagos, Santa Cruz, (one unknown sex) Española, Punta Juarez, (one unknown sex) Pinta; James, 1966 mentioned (two males, one female), Galapagos, Genovesa.
Comments
Described by Walker based on a male specimen collected by Charles Darwin in 1835. Reported for Ecuador by [Citation10,Citation11,Citation22,Citation25] based on material collected in Pinta, Fernandina, Genovesa, Española and Santa Cruz Islands. There is no material deposited in the QCAZ Museum for this species.
Distribution
Endemic to the Galapagos Archipelago [Citation10,Citation11,Citation22].
Lucilia pulverulenta Whitworth, 2014 ()
Locality records
Ecuador (two males, two females), Guayas, Balao Chico, 2.733 LS, 79.750 LW, 26/4/1963, Pena (CNC); (one female), Santo Domingo, Rio Palenque, 150 m asl, 0.583 LS, 79.366 LW, 26/4/1963, G. M. Wood.
Comments
Reported from Ecuador by Whitworth [Citation11] based on material housed at CNC Museum collection collected in Guayas and Santo Domingo Provinces in 1963. There is no material deposited in the QCAZ Museum for this species.
Distribution
This species is known from Colombia, Costa Rica, Ecuador, Honduras and Panama [Citation11].
Lucilia purpurascens (Walker, 1836) ()
Locality records
Ecuador (one male, two females), Napo, 7 km S Baeza, 2,000 m asl, Feb. 20–25, 1979, G. and M. Wood (CNC); (one male), Cuenca rd., Cañar Azuay 4 March 1965, L. Pena (CNC); (six males, eleven females), Tandapi, 40 kmSW Quito, 1300–1500 m asl, June 15–21, 1965, Pena (CNC); (one male), Napo, May/10/2002, O. Lonsdale. BNNR054, (UGG); (one male), Pichincha, DMQ Bosque Metropolitano 0.342 LS, 78.518 LW, BMS-BT05, A. Torres VSR,8/23/-9/15/2016 Bosque, A. Perez det. QCAZ 224619 (QCAZ); (nine females), Pichincha, Guajalito, 1,800 m asl, 0.216 LS, 78.750 LW, 30/5/2004, D. Paez, QCAZ 212363–71 (QCAZ); (one male), Cotopaxi, Las Pampas, 1500 m asl, 0.421 LS, 78.951 LW, 5/5/2002, G. Caroti, QCAZ 212372 (QCAZ); (one male, five females), Imbabura, Los Cedros, 1180 m asl, −0.3052778 − 78.7772, 3/8/2005, R. Cárdenas, QCAZ 212373–78 (QCAZ); (one male, two females) Zamora, Cantón El Pangui, 1413 m asl, 4 LS, 78.587 LW, 14/8/2010, A. Argoti, T. Ghia, QCAZ 212379–81 (QCAZ); (one female), Pichincha, Bellavista Ecient. 2287 m asl, 0.010 LS, 78.687 LW, 1/8/2009, R. Cárdenas, QCAZ 212382 (QCAZ); (one female), Pichincha, Sangolqui, Conocoto, 2538 m asl, 0.291 LS, 78.477 LW, 16/2/2013, C. Castro, QCAZ 212383 (QCAZ); (one male, five females), Napo, Baeza, 1949 m asl, 0.467 LS, 77.892 LW, 31/10/2015, M. Dominguez, QCAZ 212243 [MF458342], QCAZ 212244 [MF458343], QCAZ 212274 [MF458352], QCAZ 212232 [MF458355], QCAZ 212233 [MF458347], QCAZ 212270 [MF458349], (QCAZ); (two males, one female), Napo, Sarayacu, 1,312 m asl, 0.695 LS, 77.800 LW, 28/10/2015, M. Dominguez, QCAZ 212240 [MF458344], QCAZ 212241 [MF458340], QCAZ 212242 [MF458339] (QCAZ); (four females), Pichincha, Nayon, 2,397 m asl, 0.166 LN, 78.416 LW, 25/10/2013, S. Aguirre, QCAZ 114999 [MF458353], QCAZ 115000 [MF458357], QCAZ 115001 [MF458345], QCAZ 115002 [MF458348], (QCAZ); (one female), Loja, Loja, 2189 m asl, 4 LS, 79.175 LW, 17/7/2015, A. Garcia, QCAZ 212297 [MF458341] (QCAZ); (one male, 4 females), Pichincha, Quito, Parque Metropolitano, 2,542 m asl, 0.179 LS, 78.472 LW, 15/5/2015, W. Pruna, QCAZ 212245 [MF458351], QCAZ 212246 [MF458356], QCAZ 212247 [MF458354], QCAZ 212248 [MF458350], QCAZ 212249 [MF458346] (QCAZ).
Comments
Reported for Ecuador for the first time by Whitworth [Citation11] based on material housed in the CNC Museum collected from Azuay, Santo Domingo and Napo Provinces in 1965 and 1979. This species was misspelled as purpurescens in most prior publications; more nomenclatural details see [Citation11].
Distribution
L. purpurascens is considered a montane species given its distribution throughout the Andean region from 1300–1900 m asl in Peru [Citation26], 2700–3000 m asl in Colombia [Citation6], and 1200–3000 m asl in Ecuador (Pichincha, Cotopaxi, Imbabura, Zamora, Napo and Loja provinces).
Lucilia sericata (Meigen, 1826) ()
Locality records
Ecuador (one female), Pichincha, Machachi, 2900 m asl, 0.506 LS, 78.577 LW, 21iv2013, D Navarreta; (one female), Pichincha, Palmeras 24/05/92, E. Pichilingue, QCAZ 212362; (three females), Pichincha, Nayon, 2,397 m asl, 0.166 LS, 78.416 LW, 13/10/2013, S. Aguirre, QCAZ 114997 [MF458318], QCAZ 114998 [MF458319], QCAZ 114996 [MF458320] (QCAZ); (one female), Pichincha, Quito, 2,805 m asl, 0.190 LS, 78.500 LW, 30/3/2014, M. Dominguez, QCAZ 114995 [MF458321] (QCAZ); (one female), Pichincha, Nayon, 2,397 m asl, 0.166 LS, 78.416 LW, 18/10/2013, E. Moreno, QCAZ 114994 [MF458322] (QCAZ); (one female), Pichincha, Quito, 2,805 m asl, 0.190 LS, 78.500 LW, 16/7/2016, A. Torres, QCAZ 224462 (QCAZ); (two males, one female), Pichincha, Puembo, 2,592 m asl, 0.202 LS, 78.328 LW, 29/7/2016, A. Torres, QCAZ 224459–61; (one female), Pichincha, Puembo, 2,554 m asl, 0.202 LS, 78.328 LW, 29/7/2016, A. Torres, QCAZ 224463 (QCAZ); (one female), Pichincha Amaguaña, 2,618 m asl, 0.385 LS, 78.489 LW, 29/7/2016, A. Torres, QCAZ 224456 (QCAZ); (one female), Pichincha, Cashapamba, 2,684 m asl, 0.356 LS, 78.416 LW, 23/8/2016, A. Torres, QCAZ 224457 (QCAZ); (one female), Pichincha, Cerro Ilalo, 2728 m asl, 0.239 LS, 78.406 LW, 29/7/2016, A. Torres, QCAZ 224458 (QCAZ).
Comments
Reported for Ecuador based on an undetermined number of QCAZ specimens collected in Nayon in 2013 [Citation21,Citation22]. The material examined from the QCAZ Museum was collected in Orellana and Pichincha provinces. Together with L. eximia, adults of L. sericata are commonly found due to their frugivorous behavior, where immature stages can develop on rotten vegetables and fruits [Citation26]. This species has been reported as a primary and secondary myiasis producer [Citation26].
Distribution
Even though L. sericata is distributed from southern Canada to Argentina, it is not found in many areas of the Neotropics. Yet, it is known to be present in Central and South America near large cities [Citation11].
Lucilia setosa (James, 1966) ()
Locality records
Ecuador (five males, six females) paratypes, Isla Darwin, 29 January 1964, D.G. Cavagnaro (WSUP); (one female), same data except nonparatype; (two males), Española at Punta Juarez, Feb. 10–12, 1967, Ira L. Wiggins (WSUP).
Comments
James [Citation27] described L. setosa based on specimens from Darwin and Wolf islands. Reported for Ecuador by [Citation10,Citation11,Citation22,Citation25]. There is no material deposited in the QCAZ Museum for this species.
Distribution
Endemic to the Galapagos Archipelago [Citation10,Citation11,Citation22] although it is likely to occur on other pacific islands [Citation11].
Discussion
We provide evidence for the presence of eleven Luciliinae species in Ecuador: the monotypic Blepharicnema and ten species of Lucilia, from which three species, L. deceptor, L. setosa, and L. piona are endemic to the Galapagos Archipelago, and L. nitida is reported for the first time in continental Ecuador. Previous studies have reported that L. cuprina [Citation11,Citation27–30] and L. vulgata [Citation11,Citation28] (both widespread species) may be present in Ecuador according to their current distribution; however, we were not able to review any specimens for Ecuador. Aguirre [Citation21] reported the presence of Lucilia elongata (Shannon 1924) in Ecuador, a species that was later on included in Salazar and Donoso [Citation20] catalogue of species of forensic value for Ecuador. We exclude L. elongata from the present checklist because there are no previous reports in the region for this species [Citation10,Citation11] and it was likely a misidentification by Aguirre [Citation21].
To this date, Ecuadorian Calliphoridae have not been appropriately sampled, hence the diversity and distribution of Luciliinae will likely be found to be much wider than reported here. In this study, the geographical records are biased to the Andean region (particularly near to large urban settlements), while other regions in the southern Andes, Coastal region, Amazon Forest, and the Galapagos Archipelago are poorly represented (). This is a pattern commonly found in Ecuadorian invertebrates [Citation31,Citation32]. For this reason, multiregional sampling in urban (eusynanthropic), seminatural (hemysinanthropic), and natural (asynanthropic) habitats including Ecuadorian highlands and islands, is mandatory to improve available information on diversity, distribution, and ecology of these forensically important insects.
Recent research has incorporated DNA barcoding as a trustworthy method for the identification and discovery of species [Citation33,Citation34] including forensic important flies [Citation16]. Molecular identity is important to confirm species status and to contribute with a faster and more reliable analysis of the entomological evidence [Citation7,Citation16,Citation35] specially when available samples of the crime scene are immature stages, which are difficult to identify using taxonomic keys. Consequently, voucher specimens from Museum collections are highly important to confirm old records, assess taxonomic variation, and fill out gaps on distribution data [Citation28,Citation31]. Therefore, an optimal local reference collection, scientists can rely on, is hardly needed to continue with the development of forensic entomology in the country. Even though previous studies on molecular ID of forensically important flies have shown to be successful when using mitochondrial barcodes, particularly the cytochrome c oxidase subunit I- COI [Citation16,Citation24,Citation35–37], other studies have reported problems when running identity assessments [Citation38,Citation39].Barcoding, together with Museum collections, contributes with a variety of new opportunities in the analysis and interpretations of forensic importance specimens [Citation16] as well as to the biodiversity inventories and DNA data bulk of the planet [Citation34]. Finally, we expect this study to enhance the collaboration between entomologists and the legal community [Citation4,Citation7,Citation16,Citation40-42].
Acknowledgments
We are grateful with the QCAZ Museum forensic entomology research team (Washington Pruna, Saúl Aguirre, Mariela Dominguez, Fernanda Salazar, Ana Torres, Ana Belén García,Rita Hidalgo and Felipe Varela) for their collaboration in the sampling, identification and photographic process. We thank Wagner Chaves for his reviews and comments. This research was supported by Pontificia Universidad Católica del Ecuador (grants L13214 and N13456) and Fiscalía General del Estado, Ecuador (grant L14056) under the Research Permit No 003-17-IC-FAU-DNB/MA.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Courtney GW, Pape T, Skevington JH, et al. Biodiversity of Diptera. In: Foottit RG, Adlher PH, editors. Insect biodiversity science and society. Oxford: Wiley-Blackwell; 2009. p. 185–222.
- Marshall SA. Flies: the natural history and diversity of Diptera. Canada: A Firefly Book; 2012.
- Greenberg B. Flies and Disease Vol. I: ecology, classification and biotic association. Princeton, New Jersey: Defense Technical Information Center; 1971.
- Renaud AK, Savage J, Adamowicz SJ. DNA barcoding of Northern Nearctic Muscidae (Diptera) reveals high correspondence between morphological and molecular species limits. BMC Ecol. 2012;12(1):24.
- Anderson GS, Cervanka VJ. Insects associated with the body: their use and analysis. In: Haglund WD, Sorg MH, editors. Advances in forensic taphonomy: method, theory, and archeological perspectives. Florida: CRC Press; 2002. p. 173–200.
- Garcia EC. Calliphoridae (Diptera) do noroeste da América do Sul: diversidade, distribuição e código de barras genético. Manaus: Tese (Ciências Biológicas (Entomologia)) - Instituto Nacional de Pesquisas da Amazônia; 2017. p. 348.
- Moreno EA, Barragan A. Posicionamiento de la entomología forense en el Ecuador. Rev Ecuat Med Cienc Biol. 2015;36(1):41–47.
- Rivers DB, Dahlem GA. The science of forensic entomology. United States of America (USA); 2014.
- Charabidze D, Gosselin M, Hedouin V. Use of necrophagous insects as evidence of cadaver relocation: myth or reality? PeerJ. 2017;5:e3506.
- Kosmann C, Mello RP, De Harterreiten-Souza ÉS, et al. A list of current valid blow fly names (Diptera: calliphoridae) in the Americas South of Mexico with key to the Brazilian species. EntomoBrasilis. 2013;6:74–85.
- Whitworth T. A revision of the neotropical species of Lucilia Robineau-Desvoidy (Diptera: calliphoridae). Zootaxa. 2014;3810(1):1–76.
- Amat E, Velez MC, Wolff M. Illustrated key for identification to genera and species of blowflies (Diptera: calliphoridae) of Colombia. Caldasia. 2008;30:231–244.
- Norris KR. The bionomics of blow flies. Annu Rev Entomol. 1965;10:47–68.
- Sherman RA. Maggot therapy for treating diabetic foot ulcers unresponsive to conventional therapy. Diabetes Care. 2003;26:446–451.
- Moritz C, Cicero C. DNA barcoding: promise and pitfalls. PLoS Biol. 2004;2(10):e354.
- Chimeno C, Morinière J, Podhorna J, et al. DNA barcoding in forensic entomology – establishing a DNA reference library of potentially forensic relevant arthropod species. J Forensic Sci. 2019;64(2):593–600.
- Ratnasingham S, Hebert PDN. A DNA-based registry for all animal species: the Barcode Index Number (BIN) system. PloS ONE. 2013;8(7):e66213.
- Wilson JJ. DNA barcodes for insects. In: Kress WJ, DL E, editors. DNA barcodes: methods and protocols. United States: Springer; 2012. p. 17–46.
- Amat E, Wolff M. New records of Blepharicnema splendens (Calliphoridae: calliphorinae, Luciliini) from Colombia. Rev Soc Entomol Argent. 2007;66(1–2):187–190.
- Salazar F, Donoso D. Catálogo de insectos con valor forense en el Ecuador. Rev Ecuat Med Cienc Biol. 2015;36(1):47–57.
- Aguirre S. Datos preliminares de la entomofauna cadavérica en la provincia de Pichincha, Ecuador. Rev Ecuat Med Cienc Biol. 2015;36(1):67–69.
- Tantawi TI, Sinclair BJ. An update of the blow flies (Diptera: calliphoridae) of the Galapagos Islands, and first record of Chrysomya rufifacies (Macquart) from mainland Ecuador. Zootaxa. 2013;3750(3):237–250.
- Wolff M, Kosmann C. Families Calliphoridae and Mesembrinellidae. Zootaxa. 2016;4122:856–875.
- Rolo EA, Oliveira AR, Dourado CG, et al. Identification of sarcosaprophagous Diptera species through DNA barcoding in wildlife forensics. Forensic Sci Int. 2013;228(1):160–164.
- Sinclair BJ, Peck SB. An annotated checklist of the Diptera of the Galapagos Archipiélago (Ecuador). Charles Darwin Research Station. 2005;64.
- Baumgartner D, Greenberg B. Distribution and medical ecology of the blow flies (Diptera: calliphoridae) of Peru. Ann Entomol Soc Am. 1985;78:565–587.
- James MT. The blow flies of the Galapagos Islands (Diptera: calliphoridae). Proc Calif Acad Sci. 1966;34(10):475–482.
- Velásquez Y, Martínez-Sánchez AI, Thomas A, et al. Checklist and distribution maps of the blow flies of Venezuela (Diptera, Calliphoridae, Mesembrinellidae). ZooKeys. 2017;645:103–132.
- Wolff PT, Amat M. E. Los califóridos, éstridos, rinofóridos y sarcofágidos (Diptera: calliphoridae, Oestridae, Rhinophoridae, Sarcophagidae) de Colombia. Biota Colombiana. 2004;5(2):201–208.
- Salazar-Ortega J, Amat E, Gómez-Piñerez L. A checklist of necrophagous flies (Diptera, Calyptratae) from an urban area in Medellín, Colombia. Rev Mex Biodivers. 2012;83(2):562–565.
- Salazar F, Reyes-Bueno F, Sanmartin D, et al. Mapping continental Ecuadorian ant species. Sociobiology. 2015;62(2):132–162.
- Donoso DA, Salazar F, Maza F, et al. Diversity and distribution of type specimens deposited in the Invertebrate section of the Museum of Zoology QCAZ, Quito, Ecuador. Ann Soc Entomol. Fr. 2009;45(4):437–454.
- Hebert PD, Cywinska A, Ball SL. Biological identifications through DNA barcodes. Proc R Soc Lond B Biol Sci. 2003;270(1512):313–321.
- Kress W, García-Robledo C, Uriarte M, et al. DNA barcodes for ecology, evolution, and conservation. Trends Ecol Evol. 2015;30:25–35.
- Harvey ML, Dadour IR, Gaudieri S. Mitochondrial DNA cytochrome oxidase I gene: potential for distinction between immature stages of some forensically important fly species (Diptera) in western Australia. Forensic Sci Int. 2003;131(2):134–139.
- Wells JD, Wall R, Stevens JR. Phylogenetic analysis of forensically important Lucilia flies based on cytochrome oxidase I sequence: a cautionary tale for forensic species determination. Int J Legal Med. 2007;121(3):229.
- Wells JD, Williams DW. Validation of a DNA-based method for identifying Chrysomyinae (Diptera: calliphoridae) used in a death investigation. Int J Legal Med. 2007;121(1):1.
- Meier R, Shiyang K, Vaidya G, et al. DNA barcoding and taxonomy in Diptera: a tale of high intraspecific variability and low identification success. Syst Biol. 2006;55(5):715–728.
- DeBry RW, Timm A, Wong ES, et al. DNA-based identification of forensically important Lucilia (Diptera: calliphoridae) in the continental United States. J Forensic Sci. 2013;58(1):73–78.
- Keil CB. Research needs for forensic entomology in Ecuador. Rev Ecuat Med Cienc Biol. 2015;36(1):71–78.
- García-Ruilova AB, Donoso DA. Casos sin resolver y la entomología forense en Ecuador. Rev Ecuat Med Cienc Biol. 2015;36(1):59–63.
- Pruna W, Guarderas P, Donoso DA, et al. Life cycle of Lucilia sericata (Meigen 1826) collected from Andean mountains. Neotrop Biodivers. 2019;5(1):3–9.
- Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120.
- Kumar S, Stecher G, Li M, et al. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;2018(35):1547–1549.
- Stecher G, Tamura K, Kumar S. Molecular evolutionary genetics analysis (MEGA) for macOS. Mol Biol Evol. 2020;37(4):1237–1239.