ABSTRACT
A decade ago the Caribbean was almost completely uncharted in terms of intertidal ameronothroid mites. Now the present data show that these organisms are a common component of the fauna of Caribbean shorelines. Two families of Ameronothroidea are present, the Fortuyniidae with three genera and four species and the Selenoribatidae with five genera and nine species. The most common species are the fortuyniid Alismobates inexpectatus and the selenoribatid Carinozetes mangrovi, both taxa were found in the Northern Caribbean, the Greater and Lesser Antilles as well as on Central American coasts. Six species are endemic to the Caribbean, Litoribates bonairensis, L. floridae, Schusteria marina, Thalassozetes balboa, T. barbara and Thasecazetes falcidactylus. Biogeographic patterns suggest that the genera Carinozetes and Litoribates may have evolved and diversified in the Caribbean region and that the Western Atlantic Bermudian intertidal oribatid mite fauna was largely shaped by Caribbean colonizers. Most of the species found in the Caribbean are typical rock dwellers and only a minority is represented by exclusive mangrove specialists. These species are seriously threatened by the significant progressive decline of mangrove ecosystems throughout the Caribbean.
Introduction
The Caribbean region comprises vast stretches of North, Central and South American coastlines and consists of several large landmasses known as the Greater Antilles and numerous smaller Islands, namely the Lesser Antilles, the Bahamas and the Turks and Caicos. This region represents a global biodiversity hotspot harbouring high levels of endemicity in plants and animals, with each of the subareas featuring a separate biota that fits together as a biogeographic unit [Citation1]. This high biodiversity is a result of the complex and unique geographic history of the Caribbean. It is suggested that a proto-Antillean landmass existed in the middle Eocene (49–37 Mya) which became fragmented in the Oligocene (~30 Mya) and thereby formed large parts of the Greater Antilles, and the Lesser Antilles emerged sequently in the late Miocene (10–3 Mya) mostly by volcanic activity [Citation2]. These events are closely linked to the rise of the Central American Isthmus, which concluded around 2.8 Mya ago and led to the formation of two strikingly different realms, the Caribbean Sea and the eastern Pacific Ocean [Citation3]. Due to this unique geological history, the Caribbean has been of special interest for dozens of evolutionary biologists investigating vicariance and dispersal models of distribution [Citation4] whereas most studies and debates focussed on non-flying vertebrates and their possible colonization routes [Citation5]. The heightened biological interest in this area led to a comprehensive knowledge of the Caribbean fauna in general but information about some of the smallest arthropods, namely the ubiquitous oribatid mites, has been lacking for a long time.
Some decades ago, Mahunka [Citation6] stated that the greatest gaps in information about oribatid mite distribution worldwide concerned Central America and the West Indies. Subsequent studies slowly revealed the presence of numerous species at least in certain Caribbean areas. For example, Schatz [Citation7] reported 543 species to be present in Central America, Perez-Gelabert [Citation8] listed more than 30 species known from the Greater Antillean Island Hispaniola and other authors [Citation9,Citation10] reported more than 150 species mainly from the Lesser Antilles. These numbers point to a highly diverse oribatid mite fauna in the Caribbean.
However, most of these species are typical terrestrial oribatid mites dwelling in soil, leaf litter, tree trunks, canopy, etc., and only a few are also known to occur in coastal habitats. Several lohmannid species regularly occur in the littoral environment. For example, Meristacarus porcela was found in mangrove leaf litter in Guatemala [Citation7], other oribatid species, e.g. Peloribates antillensis or Cultrobates heterodactylus were also found in mangrove leaf litter in Panama [Citation7] and thus are known to show occasional incursions into the intertidal environment, but none of these taxa are exclusively intertidal organisms. Only a single species listed in the catalogue of Central American species [Citation7], namely Fortuynia yunkeri, represents a typical marine-associated species.
In the tropics there are only two oribatid mite families which have adapted to the marine littoral environment, leading a life between the tides: the Fortuyniidae, comprising four genera, Alismobates, Circellobates, Fortuynia and Litoribates, with 26 species and the Selenoribatidae, consisting of nine genera, Arotrobates, Carinozetes, Indopacifica, Psednobates, Rhizophobates, Schusteria, Selenoribates, Thalassozetes and Thasecazetes, altogether with 32 species. Although their morphology basically conforms to that of typical terrestrial taxa, these mites have longer claws to cope with tidal wave action [Citation11] and possess plastron respiration systems to breathe during tidal inundation [Citation12,Citation13]. They inhabit rocky cliffs, boulder beaches, concrete structures or mangroves where they feed on different types of intertidal algae [Citation14]. Although the above-mentioned F. yunkeri was reported in the 1950s from Central America [Citation15], it represents no Caribbean species sensu strictu because it was found on the Pacific coast of Panama. The first record of an intertidal ameronothroid mite from the Caribbean region, was that of an undetermined selenoribatid species from the Lesser Antillean St. Lucia [Citation16] and over a decade later, the first record of an undetermined fortuyniid species from the Caribbean coast of Costa Rica [Citation17] followed. These two vague reports indicated the presence of marine associated taxa but left researchers uncertain of the real distribution for more than 20 years. Pfingstl [Citation18] provided the first definitive species record from the Caribbean by describing Thalassozetes barbara from Barbados and from then on, new records and new species were published nearly every year. Pfingstl and Schuster [Citation19] showed occurrences of three genera, Alismobates, Fortuynia and Carinozetes, on coasts of Barbados and the Greater Antilles and Pfingstl et al. [Citation20] reported Alismobates inexpectatus, Carinozetes mangrovi and T. barbara from the coasts of Hispaniola. Shortly after, several new species were described, including Schusteria marina from Grenada and Martinique [Citation21], Litoribates bonairensis and Thasecazetes falcidactylus from Bonaire [Citation22], Litoribates floridae from Florida and Thalassozetes balboa from Panama and Florida [Citation23]. The most recent publication [Citation24] demonstrated a trans-Caribbean distribution of the selenoribatid C. bermudensis and C. mangrovi. In view of these recent findings it is evident that these intertidal oribatid mites are most likely a common component of the Caribbean intertidal fauna.
Most of the recent Caribbean records are a result of a three year project which yielded more yet unpublished data on the distribution of intertidal oribatid mites. Therefore, this paper aims to provide a foundation for taxonomic and biogeographic work on Caribbean intertidal mites by (1) reviewing and summarizing all existing records in literature, (2) adding yet unpublished data and compiling comprehensive up-to-date distribution information and (3) discussing biogeographic patterns.
Materials and methods
During a three-year project (2016–2019) on the biodiversity of intertidal oribatid mites, samples were collected on three field trips to different Caribbean regions. Samples of intertidal algae were scraped off the substrate (e.g. rock, mud, mangrove roots, etc.) with a knife, mostly during low tide and afterwards put in Berlese-Tullgren funnels for approximately 24 hours to extract living mites. Specimens were then picked with a fine brush and stored in absolute ethanol for transport and further investigation.
Voucher specimens from Caribbean regions are deposited in the following institutions (collection numbers are given only when provided by the museum; additionally, accession numbers for DNA-sequences archived in GenBank [https://www.ncbi.nlm.nih.gov/genbank/] are given): (a) Dominican Republic/Museo Nacional de Historia Natural “Prof. Eugenio de Jesús Marcano”, Santo Domingo, Alismobates inexpectatus (MNHNSD 08.432, 08.433), Carinozetes mangrovi (MNHNSD 08.428–08.431) and Thalassozetes barbara (MNHNSD 08.426, 08.427); (b) Barbados/Natural History Museum Vienna, T. Barbara (NHMW 21,887); (c) Bonaire/Senckenberg Museum für Naturkunde Görlitz, Litoribates bonairensis (SMNG 56,670), Thasecazetes falcidactylus (SMNG) (56,671), GenBank accession nrs. MF997501-03; (d) Panama/Museo de Invertebrados Fairchild, Universidad de Panamá, A. inexpectatus, C. mangrovi, T. balboa, GenBank accession nrs. MK035018-19, (e) Florida/US National Museum, Litoribates floridae, GenBank accession nrs. MK035001-6; (f) Martinique/Senckenberg Museum für Naturkunde Görlitz, Schusteria marina (SMNG 56,570). Voucher specimens from yet unpublished records (see ) are deposited in the collections of Senckenberg Museum für Naturkunde Görlitz, Germany and also in the collection of the Institute of Biology, University of Graz.
Table 1. Records of intertidal oribatid mite species in the Caribbean, divided into four geographic areas. Records without numbers represent yet unpublished occurrences
Table 2. Details of first Caribbean records of four different intertidal oribatid mite species. TP = Tobias Pfingstl, AL = Andrea Lienhard, GK = Gernot Kunz, HS = Heinrich Schatz
Distribution patterns are based on published literature and on numerous yet unpublished records. Apart from already published records, specimens from all Caribbean populations were determined to species level (morphospecies) to provide detailed overview of species distributions. For better orientation, the Caribbean region was partly divided into four larger geographic areas, the Greater Antilles, the Lesser Antilles, Central America and the Northern Caribbean, and these are given in the text and tables. Although not strictly Caribbean, certain records from the Pacific coast of Panama are also included in the table and the graphs. The vague Caribbean reports of Fortuyniidae gen. sp. and Selenoribatidae gen. sp [Citation16,Citation17] were added to the maps showing the distribution of families but were omitted from the maps showing the occurrence of species because original material could not be accessed and thus not be identified to species level.
For photographic documentation, a specimen was air-dried and photographed using a Keyence VHX-5000 digital microscope with automated image stacking.
Maps were created using the free and open-source vector graphics editor Inkscape (https://inkscape.org) and graphs were further processed with Adobe Photoshop 7.0.
Results
Distribution of families
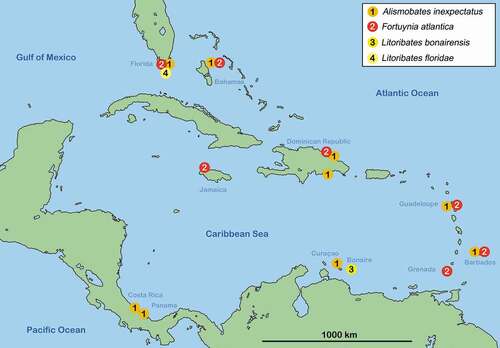
Recent research has uncovered the presence of Fortuyniidae and Selenoribatidae in the whole Caribbean region. Members of both families can be found along coastlines of the Greater and Lesser Antilles, Central America and the Northern Caribbean (, ) and show more or less consistent distributions. In Puerto Rico, Martinique and St. Lucia only selenoribatid mites have been reported so far but this may be due to low sampling activities in the respective locations. The Fortuyniidae are represented in the Caribbean by four species from three different genera, Alismobates inexpectatus, Fortuynia atlantica, Litoribates bonairensis, Litoribates floridae, and the Selenoribatidae by nine species from five different genera, namely Carinozetes bermudensis, Carinozetes mangrovi, Carinozetes trifoveatus, Schusteria marina, Selenoribates quasimodo, Selenoribates satanicus, Thalassozetes balboa, Thalassozetes barbara and Thasecazetes falcidactylus. Four of the species, F. atlantica, C. trifoveatus, S. quasimodo and S. satanicus are reported herein for the first time from the Caribbean ().
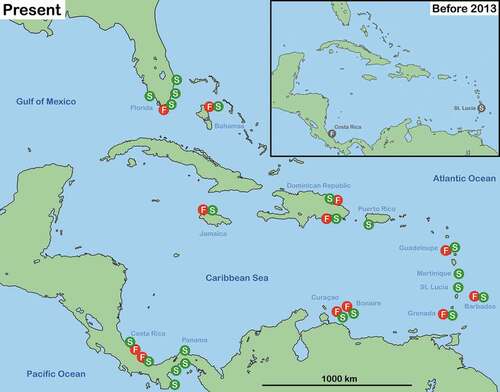
Figure 1. Graph highlighting how knowledge about the distribution of intertidal oribatid mites in the Caribbean area has changed over the last few years. Right insert showing records known before 2013; large map giving present state of knowledge for the two families occurring in this area. F = Fortuyniidae, S = Selenoribatidae
Distribution Fortuyniidae
Greater Antilles – There are relatively few records of fortuyniid species from the Greater Antilles. In the Dominican Republic A. inexpectatus and F. atlantica were found whereas the latter was only recorded from the Northern coast of the Samaná peninsula and the former was sampled from Samaná and from Boca Chica at the Southern coast (). On Jamaica, only F. atlantica is known to occur.
Lesser Antilles – Fortuynia atlantica occurs on Grenada, Guadeloupe and Barbados. Alismobates inexpectatus was also found on the latter two islands and additionally on Curaçao. From Bonaire there is only one fortuyniid species known, namely Litoribates bonairensis, which seems to be endemic for this island up to now.
Northern Caribbean – Alismobates inexpectatus und F. atlantica, both occur in Florida and the Bahamas. So far, all records made in Florida were only from the Florida Keys but here they are known from various landmasses of this chain of islands (Key Largo to Marathon). The records from the Bahamas only relate to New Providence Island, both species were found all over the island. Litoribates floridae () was only found on Islamorada, one of the islands of the Florida Keys and seems to be endemic for this area.
Central America – There are no reports of F. atlantica from Caribbean Central American coasts but A. inexpectatus was found on Isla Colón and Isla Bastimentos in Panama and on a beach in Manzanillo, Costa Rica.
Distribution Selenoribatidae
Greater Antilles – Carinozetes mangrovi is apparently widespread in the Greater Antilles with records from the Dominican Republic, Jamaica and Puerto Rico (). Thalassozetes barbara is the second selenoribatid species known from the Greater Antilles and was reported from the north and south coast of the Dominican Republic.
Figure 4. Map of the Caribbean showing occurrences of selenoribatid species in this geographic region
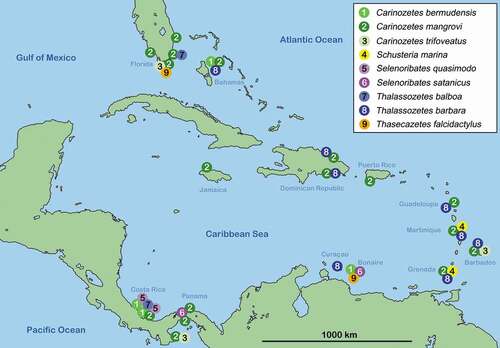
Lesser Antilles – Bonaire harbours three selenoribatid species, C. bermudensis, S. satanicus and T. falcidactylus whereas these occurrences are the only findings of these species in the Lesser Antilles. Schusteria marina was reported from Martinique and Grenada and C. trifoveatus was only found on Barbados. Carinozetes mangrovi and T. barbara show the widest distributions in the Lesser Antilles, with the former occurring on Barbados, Grenada, Guadeloupe and Martinique and the latter on Barbados, Curaçao, Grenada, Guadeloupe and Martinique.
Northern Caribbean – Again the reports from the Bahamas only relate to New Providence Island but here C. bermudensis, C. mangrovi and T. barbara were found on several locations whereas C. mangrovi was most abundant in the mangroves of South Beach and T. barbara on the rocky northern shore (e.g. Compass Point, Paradise Island). Carinozetes mangrovi, C. trifoveatus, T. balboa and T. falcidactylus are all occurring in Florida. Apart from C. mangrovi, all species were only found in single locations, C. trifoveatus and T. falcidactylus on the Florida Keys (Marathon, Islamorada) and T. balboa on Key Biscayne in Miami City. Carinozetes mangrovi, on the other hand, was found in high numbers at various locations, i.e. West Palm Beach, Miami, Florida Keys and even in Naples and Bonita Springs at the Gulf of Mexico.
Central America – The coasts of Panama are quite diverse in terms of selenoribatid species, six different species are known to occur here. Carinozetes trifoveatus was found at the Pacific coast of Panama whereas C. bermudensis, T. balboa and S. quasimodo were found at the Caribbean shoreline of Panama. Carinozetes mangrovi was the only species which was found in high numbers at various locations at both the Pacific and the Caribbean coast of Panama. Selenoribates satanicus was found on Isla Colón and was sampled from the shore of the Panama Canal approximately eight kilometres away from the open ocean which represents a quite unique finding. From the Caribbean coast of Costa Rica, so far, only two selenoribatid species are known from a single location, namely C. bermudensis and S. quasimodo.
Ecological remarks
Based on literature records and comprehensive sampling in the years 2016–2018, the Caribbean species can be classified into three ecological categories (): rock dwellers, mangrove inhabitants and euryoecious species occurring on rocks as well as in mangrove habitats. Typical rock dwellers are A. inexpectatus, F. atlantica, C. trifoveatus, S. quasimodo, T. balboa and T. barbara, all of these species were found on rocky substrates in more than 90% of all samples. Typical mangrove species are L. bonairensis, L. floridae and T. falcidactylus, whereas they were found without exception in mangrove leaf litter. Carinozetes mangrovi, C. bermudensis and S. marina are euryoecious species, they occurred nearly equally in rocky environments and mangrove habitats, whereas these mangrove habitats were mostly mangrove roots overgrown with intertidal algae. Selenoribates satanicus is difficult to classify because it was found only in two samples, once in mangrove leaf litter and once in leaf litter from the shore of the Panama Canal which does not represent a mangrove habitat.
Table 3. Occurrences in different environments (rocky and mangrove habitats) of the Caribbean species based on 109 samples taken in the years 2016–2018. R – rock dweller; E – euryoecious species; M – mangrove dweller
With regard to the ecological classification, species belonging to the same group, were often found together at the exact same location. In nearly 50% of all samples at least two species occurred syntopically, in less than 10% a maximum of three species was found in the same patch of algae. Alismobates inexpectatus and T. barbara were found most frequently together, followed by A. inexpectatus and F. atlantica. Carinozetes trifoveatus, T. balboa and T. barbara, on the other hand, never occurred syntopically.
Discussion
Apart from some terrestrial oribatid mite species that were occasionally found in Caribbean littoral environments, for example Meristacarus porcela in Guatemala and Peloribates antillensis and Cultrobates heterodactylus, both in Panama [Citation7], the Caribbean intertidal environment was almost completely uncharted in terms of intertidal oribatid mites before 2013, and it was unknown if these mites do really exist there. The present data clearly confirm the occurrence of Fortuyniidae and Selenoribatidae in the Caribbean and show that these families are distributed throughout this region representing a common component of the local intertidal fauna. There are still large gaps in the distribution, for example along the South American shoreline, Cuba, northern Central America, which are without a doubt caused by the lack of sampling activities in these areas.
Most of the genera present in the Caribbean can be found in the subtropics and tropics all over the world but Carinozetes, Litoribates and Thasecazetes are so far confined to the Caribbean and adjacent regions, namely the Western Atlantic and the Eastern Pacific [Citation25,Citation26], suggesting the evolutionary origin of these taxa in these regions. Pfingstl and Lienhard [Citation21] already stated that Carinozetes may be derived from an ancestral Western Atlantic Schusteria clade and the present data support this assumption. Apart from S. marina in the south-western area of the Caribbean, Schusteria is completely lacking in this region whereas Carinozetes is present throughout the Caribbean which indicates that this genus most likely evolved and diversified in this area. The records of Carinozetes from the Eastern Pacific further show that the genus has evolved and spread long before the closure of the Central American Isthmus approximately 2.8 mya ago [Citation3]. A similar evolutionary scenario is assumable for Litoribates as its absence from Western Atlantic areas suggests an Eastern Pacific ancestry. The monotypic Thasecazetes, on the other hand, is restricted to the Caribbean but records are yet so scarce that it is impossible to make any assumptions concerning its biogeographic history.
Prior to this study, many of the present species were only known from Bermuda in the Western Atlantic, and based on the young geological age of this archipelago it was assumed these species are derived from populations somewhere in the Caribbean [Citation27]. Bermuda is situated in the Gulf Stream and intertidal oribatid mites are thought to be mainly transported between landmasses by drifting along ocean currents [Citation27]. Transport along the Gulf Stream from North- and Central America to Bermuda has been hypothesized for several terrestrial lohmannid species, for example Lohmannia similis and Meristacarus porcela, two mite species also known to often occur in the littoral zone [Citation28]. Moreover, based on experimentally inferred survival times, it was demonstrated that Fortuynia atlantica and Carinozetes bermudensis theoretically could survive transport along the Gulf Stream from Central America to Bermuda [Citation27]. The present data confirm this biogeographic link and suggest that the Bermudian intertidal mite fauna was largely shaped by Caribbean colonizers. It is likely that Caribbean ocean currents and gyres play an important role in the dispersal of these coastal associated organisms but final evidence is still lacking.
However, in Fortuyniidae and Selenoribatidae there seem to be species with wide distribution areas and only a few with strongly limited occurrences. The fortuyniid A. inexpectatus and F. atlantica and the selenoribatid C. mangrovi and T. barbara show a trans-Caribbean occurrence indicating high dispersal potential and most other species show either a disjunct distribution or at least a wider distribution in larger geographic areas of the Caribbean. Only Litoribates bonairensis and L. floridae apparently represent short-range endemics as they were found only in a single location. But this picture may be misleading because some of the more widely distributed species were shown to consist of distinct genetic lineages and some of the lineages show smaller distribution areas. For example, Carinozetes mangrovi possesses a northern lineage and an Antillean lineage [Citation24]. Indeed, there are indications that some of the other widely distributed morphospecies, e.g. A. inexpectatus, F. atlantica and T. barbara, may also consist of geographically restricted genetic lineages, possible cryptic species respectively, as indicated by preliminary molecular genetic investigations (unpublished data) but until such patterns are finally verified further discussion is premature.
From an ecological point of view, it is interesting that the majority of species represent rock dwelling taxa and only a minority are exclusive mangrove specialists. The cause for this disparity is unknown and more comprehensive data is necessary to draw conclusions in this respect, but increasing mangrove degradation and deforestation may play a role. Caribbean mangroves have declined by approximately 24% over the last quarter century as a result of coastal development and human exploitation [Citation29]. Indeed, deforestation rates in mangroves are four times larger than those in terrestrial tropical rainforests [Citation30] and this may already have caused several mangrove associated species to become extinct in the Caribbean area, especially those on smaller islands. Moreover, climate change and its consequences are expected to cause an additional significant decline of mangrove ecosystems throughout the Caribbean [Citation29] which will most likely further reduce the diversity of mangrove dwelling intertidal oribatid mite species.
Despite the present data, knowledge about the intertidal oribatid mite fauna of the Caribbean is still incomplete and thus we cannot estimate consequences of mangrove loss or other threats. Further comprehensive studies are necessary to understand the evolutionary history and to predict possible future scenarios for these organisms in this region.
Acknowledgments
I want to thank various people that somehow contributed to this publication. First of all, thanks to R. Schuster for his support during the initial phase of Caribbean research and to A. Lienhard for her help in collecting most of the Caribbean material. Thanks also to H. Schatz for giving very helpful information and advices and for providing additional specimens from Bonaire, and thanks to G. Kunz for providing specimens from Costa Rica. I am also grateful to J. Baumann for drawing and providing a blank map of the Caribbean which was used for creating the present graphs. Thanks to all Caribbean authorities for permits, support and sometimes even accommodation. This investigation was funded by the Austrian Science Fund (FWF): [P 28597].
Disclosure statement
The author has no conflicts of interest to disclose.
Additional information
Funding
References
- Myers N, Mittermeier RA, Mittermeier CG, et al. Biodiversity hotspots for conservation priorities. Nature. 2000;403(6772):853–858.
- Iturralde-Vinent MA. Meso-Cenozoic Caribbean paleogeography: implications for the historical biogeography of the region. Int Geol Rev. 2006;48(9):791–827.
- Fortunato H. The Central American land bridge: evolution at work. Schrift naturwiss Verein Schleswig-Holstein. 2008;70:56–72.
- Mayr E. Geographic speciation in tropical echinoids. Evol. 1954;8(1):1–18.
- Ricklefs R, Bermingham E. The West Indies as a laboratory of biogeography and evolution. Phil Trans Biol Sci. 2008;363(1502):2393–2413.
- Mahunka S. Neue und interessante Milben aus dem Genfer Museum. XLIV. Oribatida Americana 5: costa Rica (Acari). Arch Sci (Genève). 1982;35:179–193.
- Schatz H. Catalogue of known oribatid mites species (Acari, Oribatida) from Central American landbridge (First part). Trop Zool. 2006;19:209–288.
- Perez-Gelabert DE. Arthropods of Hispaniola (Dominican Republic and Haiti): a checklist and bibliography. Zootaxa. 2008;1831(1):1–530.
- Ermilov S, Smit H. Additions to the oribatid mite fauna of the Caribbean, with a description of a new species of Epidamaeus (Acari, Oribatida, Damaeidae). Acarologia. 2017;57:791–804.
- Niedbała W, Ermilov S. New species of ptyctimous mites (Acari, Oribatida) from the Caribbean. Syst Appl Acarol. 2017;22:241–252.
- Pfingstl T, Kerschbaumer M, Shimano S. Get a grip – evolution of claw shape in relation to microhabitat use in intertidal arthropods (Acari, Oribatida). PeerJ. 2020;8:e8488:14.
- Pugh PJA, King PE, Fordy MR. Respiration in Fortuynia maculata Luxton (Fortuyniidae: cryptostigmata: acarina) with particular reference to the role of van der Hammen’s organ. J Nat Hist. 1990;24(6):1529–1547.
- Pfingstl T, Krisper G. Plastron respiration in marine intertidal oribatid mites (Acari, Fortuyniidae and Selenoribatidae). Zoomorph. 2014;133(4):359–378.
- Pfingstl T. The marine-associated lifestyle of ameronothroid mites (Acari, Oribatida) and its evolutionary origin: a review. Acarologia. 2017;57:693–721.
- Van Der Hammen L. Description of Fortuynia yunkeri nov. spec., and notes on the Fortuyniidae nov. fam. Acarologia. 1963;5:152–167.
- Schuster R. Die Selenoribatidae, eine thalassobionte Familie der Hornmilben (Oribatei). Acarologia. 1977;19:155–160.
- Schuster R. Transoceanic distribution of air-breathing littoral mites. Progr Acarol. 1989;1:355–362.
- Pfingstl T. Thalassozetes barbara n. sp. (Acari, Oribatida), a new intertidal species from the coast of Barbados. Acarologia. 2013;53(4):417–424.
- Pfingstl T, Schuster R. Global distribution of the thalassobiontic Fortuyniidae and Selenoribatidae (Acari, Oribatida). Soil Org. 2014;86:125–130.
- Pfingstl T, De Los Santos G, Lienhard A. First records of intertidal mite species (Acari: Acariformes: Oribatida) from Hispaniola’s coasts with two new records for the Caribbean. Rev Iber Aracn. 2016;29:41–44.
- Pfingstl T, Lienhard A. Schusteria marina sp. nov. (Acari, Oribatida, Selenoribatidae) an intertidal mite from Caribbean coasts, with remarks on taxonomy, biogeography, and ecology. Int J Acarol. 2017;43(6):462–467.
- Pfingstl T, Baumann J, Lienhard A, et al. New Fortuyniidae and Selenoribatidae (Acari, Oribatida) from Bonaire (Lesser Antilles) and morphometric comparison between Eastern Pacific and Caribbean populations of Fortuyniidae. Syst Appl Acarol. 2017;22:2190–2217.
- Pfingstl T, Lienhard A, Baumann J. New and cryptic species of intertidal mites (Acari, Oribatida) from the Western Caribbean – an integrative approach. Int J Acarol. 2019;45(1–2):10–25.
- Pfingstl T, Baumann J, Lienhard A. The Caribbean enigma: the presence of unusual cryptic diversity in intertidal mites (Arachnida, Acari, Oribatida). Org Div Evol. 2019;19(4):609–623.
- Pfingstl T, Schuster R. Carinozetes nov. gen. (Acari: oribatida) from Bermuda and remarks on the present status of the Family Selenoribatidae. Acarologia. 2012;52(4):377–409.
- Pfingstl T, Schatz H. New littoral mite species (Acari, Oribatida, Fortuyniidae) from the Galápagos archipelago with ecological and zoogeographical considerations. Zootaxa. 2017;4244(1):39–64.
- Pfingstl T. Resistance to fresh and salt water in intertidal mites (Acari: oribatida): implications for ecology and hydrochorous dispersal. Exp Appl Acarol. 2013;61(1):87–96.
- Schatz H, Schuster R. First records of Lohmanniidae (Acari: oribatida) from the Bermuda Islands. Acarologia. 2012;52(3):247–257.
- Wilson R. Impacts of climate change on mangrove ecosystems in the coastal and marine environments of Caribbean Small Island Developing States (SIDS). Sci Rev. 2017;61–82.
- Blanco JF, Estrada EA, Ortiz LF, et al. Ecosystem-wide impacts of deforestation in mangroves: the Urabá Gulf (Colombian Caribbean) case study. ISRN Ecol. 2012;2012 Article ID 958709, 14p. 1–14.