ABSTRACT
Identifying patterns of herpetofaunal diversity is imperative to the conservation of these species and the ecosystems on which they rely. In this study, we determined species occurrence and composition of the herpetofauna at the Reserva de Biósfera Estación Biológica del Beni and the Chimane Reserve Indigenous Territory. Combined data resulted in documentation of 97 species belonging to 22 families and 50 genera. Reptiles were represented by 14 families and 47 species, while amphibians were represented by eight families and 50 species. This study provides a robust inventory and report of the herpetofauna at this site and delivers data that can be used in future studies. Our results indicate that this site harbors many species, which likely contributes to the collective maintenance of biodiversity in the Bolivian lowlands. Conservation initiatives should consider the collective uniqueness of vertebrate communities maintained by habitat mosaics in this area and strive to implement strategies that preserve and manage landscape-level biodiversity.
KEYWORDS:
Introduction
Knowledge of characteristics such as species richness, assemblage, and diversity is essential to the identification of community stability, biodiversity patterns, and identification of biodiversity hotspots. These are key components of successful science-based conservation; therefore, studies that document these characteristics are critical.
Reptiles and amphibians are considered two of the most threatened vertebrate groups worldwide, yet they are also among the most poorly understood [Citation1,Citation2]. Identifying patterns of herpetofaunal diversity is imperative to the conservation of these species and the ecosystems on which they rely. Herpetofauna are particularly vulnerable to major climate and land use changes, such as forest fragmentation, habitat loss, and overall community disruption – all threats of global significance [Citation1,Citation3]. However, herpetofaunal communities are also known to vary across natural changes in the biotic and abiotic environment. Therefore, herpetofaunal composition and structure are thought to be complex; hence, further study is essential to fully elucidate these patterns and processes, especially in poorly studied areas.
Most Neotropical studies focused on herpetofauna have been conducted at sites in Brazil [Citation4,Citation5], Colombia [Citation6,Citation7], Ecuador [Citation8,Citation9], Mexico [Citation10,Citation11], and Peru [Citation12,Citation13]. However, very few studies have investigated these topics in Bolivia, especially in the Amazonian lowland regions. Bolivia is a major biodiversity stronghold that presents unique and exceptional research opportunities for biologists, especially in regard to herpetology [Citation14–18]. The mega-diversity present in Bolivia is attributed to the extensive heterogeneity of factors such as elevation and geography [Citation14,Citation18]. Although rich in herpetofaunal diversity, Bolivia lacks even basic foundational surveys on species occurrence in a majority of the habitats and ecosystems throughout the country. This lack of biological knowledge poses a major challenge to conservation. Steinberg [Citation19] and Moravec and Aparicio [Citation20] have attributed much of this lack of biological knowledge of Bolivia to the extreme inaccessibility of many parts of the country. Despite this, a few studies and surveys have contributed a large majority of the available knowledge on Bolivian herpetofauna (e.g. [Citation21,Citation22,Citation23,Citation24]). However, knowledge of species occurrence, abundance, and overall distribution of Bolivian herpetofauna is incomplete, which poses a major challenge to effective conservation and overall environmental management not only in Bolivia, but throughout many parts of South America [Citation18,Citation25,Citation26]. Without the basic elements or foundational understanding of these parameters, predicting vulnerability to change and implementing conservation strategies is difficult.
Based on the aforementioned need for baseline data and surveys in Bolivia, the objectives of our study were to survey, characterize, and report on the herpetofauna of the Reserva de la Biósfera Estación Biológica del Beni and the nearby Chimane Reserve Indigenous Territory. We aimed to provide information on species occurrence, community composition, and overall structure of the herpetofauna at this site.
Materials and methods
Study site
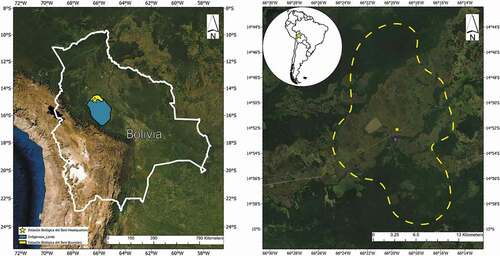
The Reserva de la Biósfera Estación Biológica del Beni (EBB; −14.868260, −66.326272; datum WGS84; ) and the Chimane Reserve Indigenous Territory (CR; −14.882, −66.328; datum WGS84; ) are near the town of San Borja in the Department of Beni, Bolivia. Although seemingly two separate entities, the two land holdings essentially form one large area of available habitat and refuge in the northwestern part of the country. These sites are located within the provinces of General José Ballivián and Yacuma, Bolivia. Due to the wet and warm weather, this area is commonly described as a tropical monsoon or tropical wet climate, as per the Köppen Climate Classification System [Citation27]. Elevation is low (220 m) and the landscape is characterized by a diversity of habitats including primary tropical rainforest, secondary tropical rainforest, tertiary forests, grassland savannas (which are considered the most species-rich of all grassland ecosystems throughout the entirety of South America; [Citation28,Citation29]), forest islands, and wetlands [Citation30,Citation31]. The climate is typical of the Bolivian Amazon Basin, with a mean annual temperature of 26°C, rainfall ≥190 cm, and humidity ≥75% [Citation31]. The EBB is managed and operated by Servicio Nacional de Áreas Protegidas (commonly referred to as SERNAP). The nearby community of Totaizal is comprised of ~150 people primarily of the Chimane tribe and is located within the Chimane Reserve and Indigenous territory [Citation32].
Figure 1. (A.) Map of Bolivia indicating boundaries of protected areas included in this study. (B.) Map of sampling area (dashed yellow line) at the Reserva de la Biósféra Estación Biológica del Beni (yellow star) and the Chimane Reserve Indigenous Territory (magenta star). Inset map indicates location in Bolivia and South America
Herpetofaunal surveys
We assessed herpetofaunal species occurrence by conducting visual encounter surveys along non-fixed transect routes during June-August from 2014–2017. Each survey lasted 4–6 hrs. We traversed the area on foot, and nighttime searches were aided by the use of headlamps (~350 lumens) and handheld flashlights (~300 lumens). Specimens were captured using hooks, tongs, or by hand. Captured individuals were measured and weighed, and these data were subsequently recorded. Following these measurements, we identified sex and photographed each individual following the methodology of Eversole et al. [Citation33]. Each specimen was then deposited into the herpetology collection of Centro de Investigación de Recursos Acuáticos (CIRA) at Universidad Autónoma del Beni “José Ballivián” in Trinidad, Bolivia [Citation33]. We followed the taxonomic authority of the Integrated Taxonomic Information System (Retrieved 30 October 2020, from the Integrated Taxonomic Information System (ITIS) (http://www.itis.gov)). We identified six general habitat types across the landscape mosaic within our study areas that included forest islands (FI), grasslands (GL), wetlands (WL), primary rainforest (PF), secondary rainforest (SF), and tertiary rainforest (TF). Based on these classifications, we recorded the habitat type from which each specimen was collected.
Data analysis
We calculated diversity using the Simpson’s index, which incorporates species richness and evenness into the calculation and better reflects natural structure of the herpetofaunal community. We chose this index because it is considered one the most meaningful and robust diversity indices available [Citation34]. To represent evenness, we created a Whittaker’s rank abundance plot. We excluded the data collected at this site by Middendorf and Reynolds [Citation31] in quantitative analyses by habitat type because species abundance data per habitat were unavailable. However, we did include this data in the overall list of species occurrence to provide a comprehensive overview of species recorded at this site and to improve the accuracy of our report of the herpetofauna for this area. We performed a nonparametric Kruskal-Wallis ANOVA to test for differences in species richness and diversity between habitat types using year as a replication.
Results
We documented 97 species (Middendorf and McReynolds [Citation31] and our study combined; 71 species from our study, 26 species unique to Middendorf and McReynolds [Citation31]) representing 22 families and 50 genera (). Of these, five were identifiable only to genus and likely represent poorly described or novel species (). Reptiles were represented by 14 families and 47 species, while amphibians were represented by eight families and 50 species ().
Table 1. List of herpetofaunal species documented at the Reserva de la Biósfera Estación Biológica del Beni and the Chimane Reserve Indigenous territory and their IUCN status. Species rows with X from Middendorf and Reynolds [Citation31]. *Phrynops geoffroanus only recorded as present, no habitat data available
Herpetofaunal species were found in each general habitat type (; ). Wetland habitats had the largest number of unique species (i.e. species found only in that particular habitat type), followed by SF, PF, GL, TF, and FI, respectively (). The Whittaker plot for combined species indicated variable evenness, demonstrating that some species can be considered abundant and common, while others can be considered rare and uncommon at this site (; ).
Figure 2. Total species richness for combined species (i.e. reptiles and amphibians), reptiles, amphibians, and unique species in forest island (FI), grassland (GL), wetland (WL), primary forest (PF), secondary forest (SF), and tertiary forest (TF) habitats
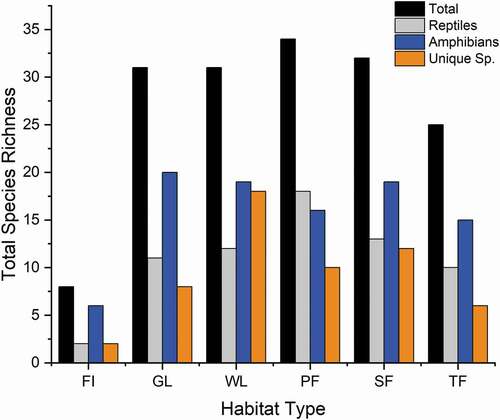
Figure 3. Whittaker plot of total combined species evenness for herpetofauna sampled at Reserva de la Biósfera Estación Biológica del Beni and the Chimane Reserve Indigenous Territory
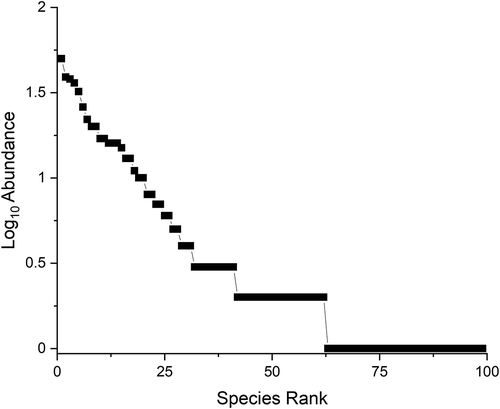
Figure 4. Simpson’s diversity index values (±SE) for (A.) total combined species, (B.) amphibians, and (C.) reptiles in forest island (FI), grassland (GL), wetland (WL), primary forest (PF), secondary forest (SF), and tertiary forest (TF) habitats
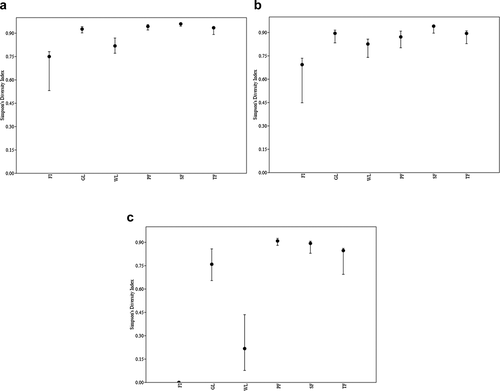
Combined species richness (H = 7.6; P = 0.2), reptile species richness (H = 3.7; P = 0.6), and amphibian species richness (H = 5.1; P = 0.4) did not differ between habitat types (). However, the number of unique species was significantly different between habitats (H = 13.0; P = 0.02; α = 0.05; ). Simpson’s diversity indices for each habitat type ranged from 0.75 to 0.96 for combined species, 0.69 to 0.94 for amphibians, and from 0 to 0.9 for reptiles (). Amphibian diversity (H = 21.5; P < 0.001), reptile diversity (H = 21.9; P < 0.001), and overall diversity (H = 19.8; P = 0.001) differed among habitat types ().
Discussion
Herpetofauna are considered among the most threatened terrestrial vertebrates worldwide [Citation1]; however, baseline information such as species presence, composition, and diversity are particularly lacking [Citation11]. This is especially true for neotropical ecosystems, specifically in Amazonian Bolivia. These data demonstrate that this site hosts a large amount of herpetofaunal diversity. Past literature indicates that EBB is an important protected area for maintaining neotropical biodiversity, especially in regard to herpetofauna [Citation28,Citation29,Citation31]. Our study supports these conclusions and indicates that a large amount of the herpetofaunal species diversity is also maintained within the CR, which was not extensively sampled in past studies conducted in this area.
Bolivian tropical forests are located at the southernmost portion of the range of this habitat type; therefore, it has been postulated that herpetofaunal diversity is less than that of more northerly sites [Citation31]. Although our data show a large amount of herpetofaunal diversity at EBB and CR, they support and coincide with this hypothesis. Another general hypothesis of the mega-diversity present in this part of the Neotropics, is that the occurrence of extreme habitat diversity (e.g. tropical rainforests, swamp forests, savanna forests, seasonally dry forests, savannas, lagoons, lakes, and rivers) results in numerous species assemblages merging in this area; therefore, herpetofaunal diversity could be equal to, or greater than, that of other areas of South America [Citation35,Citation36]. Moreover, Laurencio and Malone [Citation37] stated that herpetological diversity at transition zones in Central America was high because characteristic species of all habitat types are present and meet in these areas. Our results support these hypotheses and further demonstrate similar ecological and community dynamics across habitat mosaics. This should be a major consideration in conservation planning, applied management, and most importantly, sustainable use practices (i.e. forest resource extraction, grazing, etc.).
Our data demonstrate that this site hosts many species that occur in low abundances or can be considered rare. For example, a large majority of the species encountered were represented by a single specimen (). Although this is common in community studies, especially those focused on herpetofauna, this demonstrates this site’s importance in regards to maintenance of biodiversity in this region. This is likely why we observed statistically significant differences in diversity (includes abundance data) but not species richness between habitat types. The differences observed between broad-scale habitat types (e.g. grassland, wetland, and forest types) relative to diversity may demonstrate a natural spectrum or change in the herpetofaunal community across the landscape mosaic as a result of niche specialization and life history requirements. The sensitivity of reptiles and amphibians to habitat changes (both natural and human induced) is known to be highly variable among species and largely tied to life history traits and overall habitat requirements. For example, each habitat retained a suite of species that were unique to the particular habitat type where they were found. Interestingly, the most complex habitat types (wetlands, primary forest, and secondary forest) hosted the largest numbers of unique species. However, it is unclear why secondary forest hosts a large number of unique species not found in any other habitat type. It’s possible that we may have observed a combination of landscape environmental change with trade-offs between growth capacity and low-resource tolerance [Citation38].
It is important to note that our results may not completely reflect the true amount of herpetofaunal species richness and diversity at this site because our sampling was constrained to the dry season months (June-August). Middendorf and Reynolds [Citation31] did conduct surveys during the wet season and noted that the number of species encountered did differ between seasons. Unsurprisingly, they encountered more amphibians during the wet season. However, they did note that their collection efforts and data were lacking during potentially important months of the year as well (December-June; August-October). Although we have added a number of previously undocumented species for this site, it is likely that intensive surveys during the wet season at EBB and CR would likely yield a suite of species that have yet to be recorded. As stated by Middendorf and Reynolds [Citation31], due to the incomplete knowledge of species occurrence and diversity, it is both difficult and inappropriate to compare the relative diversity of habitat types at this site with other Amazonian regions.
Conservation implications
The remarkable diversity of ecosystems present at EBB and in the CR likely provide many niche and habitat types to support a high herpetofaunal diversity. Data on species occurrence and abundance are becoming increasingly important in contemporary biological research, especially as it relates to conservation. Studies that focus on species inventory and documentation have been noted as fundamental in designating conservation areas and advocating for extended and continued protection of nature reserves [Citation39] This is especially important due to the ever-increasing anthropogenic effects on natural resources and biodiversity across remote areas of the developing world (e.g. Bolivia; [Citation33]). Furthermore, as stated by Rocha et al. [Citation39], it is estimated that ≥86% of earth’s species remain unknown; therefore, scientists should strive to rigorously document biodiversity through carefully planned collections in order to effectively preserve and understand it. Therefore, increasing our understanding of species occurrence is necessary for scientific advancement and biological conservation. Records of species occurrence and knowledge of biodiversity patterns are essential in assessing land-use and climate change effects on vertebrates and the habitats that they occupy, particularly in areas of extreme levels of biodiversity, such as the Neotropics. Bolivian ecosystems, in particular the habitat mosaics present in the Beni department, maintain a large amount of neotropical biodiversity. However, Bolivia, like many neotropical sites, is extremely underserved in regards to biodiversity research and monitoring, especially in comparison to other parts of the world [Citation33]. Therefore, continued and future research is greatly needed to fully document, understand, and maintain this incredible amount of biodiversity, which has major implications in global ecology and conservation.
This study adds to the collective knowledge of Bolivian herpetofaunal occurrence and diversity in an understudied region of the country. By incorporating and expanding upon our findings, these efforts can be greatly improved upon at both local and global scales.
Acknowledgments
Funding and logistical support was provided by Texas A&M University—Kingsville, Texas A&M International University, Centro de Investigación de Recursos Acuáticos, and Universidad Autónoma del Beni “Jośe Ballivián”, particularly Luis Carlos Zambrano Aguirre, Jesus Egez Rivero, and Federico Moreno Aulo. We thank Servicio Nacional de Áreas Protegidas, Reserva de la Biósfera Estación Biológica del Beni, particularly Carola Vaca and Tito Zelada, as well as the community of Totaizal for field assistance and access to study areas. We thank Alexandra L. Brenk for editorial comments on previous versions of this manuscript. Specimen collection was approved by the TAMUK (#2018-05-22) and TAMIU (#2018-3) Animal Care and Use Committee and permitted by the Dirección General de Biodiversidad y Áreas Protegidas de Bolivia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Gibbons JW, Scott DE, Ryan TJ, et al. The global decline of reptiles, déjà vu amphibians. BioScience. 2000;50(8):653–666.
- Stuart SN, Chanson JS, Cox NA, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306(5702):1783–1786.
- Wang J, Liu LYH, Martin K, et al. Implications of continuous amphibian diversity monitoring in Daweishan national nature reserve in tropical SE Yunnan, China. Global Ecol Conserv. 2019;20:e00694.
- Oliveira BF, São-Pedro VA, Santos-Barrera G, et al. AmphiBIO, a global database for amphibian ecological traits. Sci Data. 2017;4(1):17123.
- Saccol SSA, Bolzan AMR, Gomes Dos Santos T. In the shadow of trees: does Eucalyptus afforestation reduce herpetofaunal diversity in Southern Brazil? S Am J Herpetol. 2017;12(1):42–56.
- Brüning LZ, Krieger M, Meneses-Pelayo E, et al. Land-use heterogeneity by small-scale agriculture promotes amphibian diversity in montane agroforestry systems of Northeast Colombia. Agr Ecosyst Environ. 2018;264:15–23.
- Rojas-Morales JA, Marin-Martínez M. Diversity, structure and natural history of amphibians in the upper Claro river basin, a buffer zone of the national natural park Los Nevados, central Cordillera of Colombia. J Threat Taxa. 2019;11(3):13261–13277.
- Tolhurst BA, Penafiel VA, Mafla-Endara P, et al. Lizard diversity in response to human-induced disturbance in Andean Ecuador. Herpetol J. 2016;26:33–39.
- Torres-Carvajal O, Pazmiño-Otamendi G, Salazar-Valenzuela D. Reptiles of Ecuador: a resource rich portal with a dynamic checklist and photographic guides. Amphib Reptile Conserv. 2019;13:209–229.
- Cruz-Elizalde R, Ramírez-Bautista A, Hernández-Ibarra X, et al. Species diversity of amphibians from arid and semi-arid environments of the real Guadalacázar state reserve, San Luis Potosí, Mexico. Nat Area J. 2016;36(3):302–309.
- Russildi G, Arroyo-Rodríguez V, Hernández-Ordónez O, et al. Species- and community-level responses to habitat spatial changes in fragmented rainforests: assessing compensatory dynamics in amphibians and reptiles. Biodivers Conserv. 2016;25(2):375–392.
- Duellman WE, Venegas PJ. Diversity of marsupial frogs (Anura: Hemiphractidae: Gastrotheca) in the northern Cordillera central, Peru, with the descriptions of two new species. Phyllomedusa: Journal of Herpetology. 2016;15(2):103–117.
- Villacampa J, Whitworth A, Allen L, et al. Altitudinal differences in alpha, beta and functional diversity of an amphibian community in a biodiversity hotspot. Neotrop Biodivers. 2019;5(1):60–68.
- Kӧhler J, Price AH. New species of Eleutherodactylus (Anura: Leptodactylidae) from cloud forest of Bolivia. Copeia. 2000;2000(2):516–520.
- Reichle S, Embert D. New species of Clelia (Colubridae) from the inter-Andean dry valleys of Bolivia. J Herpetol. 2005;39(3):379–383.
- Ocampo M, Aguilar-Kirigin A, Quinteros AS. A new species of Liolaemus (Iguania: Liolaemidae) of the Alticolor group from La Paz, Bolivia. Herpetologica. 2012;68(3):410–417.
- Embert D, Reichle S, Larrea-Alcázar DM, et al. Priority areas for amphibian conservation in a neotropical megadiverse country: the need for alternative, non place based, conservation. Biodivers Conserv. 2011;20(7):1557–1570.
- De La Riva I, Reichle S. Diversity and conservation of the amphibians of Bolivia. Herpetol Monogr. 2014;28(1):46–65.
- Steinberg P. Environmental leadership in developing countries: transnational relations of biodiversity policy in Costa Rica and Bolivia. Cambridge (MA): MIT Press; 2001.
- Moravec J, Aparicio J. Notes on the herpetofauna of Nacebe (Provincia Abuna, Departamento Pando, Bolivia). J Nat Mus Prague, Nat Hist Series. 2004;173:13–28.
- De La Riva I. Lista preliminar de los anfibios de Bolivia con datos sobre su distribución. Bollettino Museo Regionale di Scienze Naturali Torino. 1990;8:261–319.
- Dirksen L, De La Riva I. The lizards and amphisbaenians of Bolivia (Reptilia, Squamata): checklist, localities, and bibliography. Graellsia. 1999;55:199–215.
- De La Riva I, Kohler J, Lotters S, et al. Ten years of research on Bolivian amphibians: updated checklist, distribution, taxonomic problems, literature and iconography. Revista Española de Herpetología. 2000;14:19–164.
- Embert D Distribution, diversity and conservation status of Bolivian reptiles [dissertation]. Bonn, (GER): Rheinischen Friedrichs-Wilhelms-Universitat; 2007.
- Smith MF, Patton JL. Diversification of South American murid rodents: evidence from mitochondrial DNA sequence data for the akodontine tribe. Biol J Linn Soc. 1993;50(3):149–177.
- Margules CR, Pressey RL. Systematic conservation planning. Nature. 2000;405(6783):243–253.
- Kottek M, Grieser J, Beck C, et al. World map of the Koppen-Geiger climate classification updated. Meteorol Z. 2006;15(3):259–263.
- Reichle S. Frösche des Savaimeiigebitós àes Estación del Béni (EBB), Bolivien. Teil II: die Familien Leptodactylidae, Microhylidae und Hylidae (l. TeiI). Herpetofauna. 1997a;19:11–18.
- Reichle S. Frösche des Savaimeiigebitós àes Estación del Béni (EBB), Bolivien. Teil III: die Familien Hylidae (Teil 2), Schlubetrachtung und Literatur. Herpetofauna. 1997b;19:11–18.
- Flores EI. Perfil ornitológico de la Reserva de la Biosfora “Estacion Biológica Béni”. Museo Nacional de Historia Natural (Bolivia), Comunicacion. 1988;8:7–14.
- Middendorf G, Reynolds R. Herpetofauna of the Beni biological station biosphere reserve, Amazonian Bolivia: additional information, and current knowledge in context. In: Herrera-macbryde O, Dallmeier F, MacBryde B, et al., editors. Biodiversity, conservation and management in the region of the Beni biological station biosphere reserve, Bolivia. Washington (D.C.): Smithsonian Institution; 2000. p. 151–169.
- Chicchon A Chimane resource use and market involvement in the Beni Biosphere Reserve, Bolivia [dissertation]. Gainesville (FL): University of Florida; 1992.
- Eversole CB, Powell RL, Lizarro D, et al. Introduction of a novel natural history collection: a model for global scientific collaboration and enhancement of biodiversity infrastructure with a focus on developing countries. Biodivers Conserv. 2019;28(7):1921–1931.
- Magurran AE. Measuring biological diversity. Malden (MA): Blackwell Publishing; 2004.
- Foster RB, Parker III TA, Gentry AH, et al. The Tambopata-Candamo reserved zone of southeastern Peru: a biological assessment. Washington, D.C.: Conservation International (Rapid Assessment Program Working Pap. 6). 1994.
- Gentry AH, León B. Tambopata region, Peru. In: Davis SD, Heywood VH, Herrera-macbryde O, et al., editors. Centres of plant diversity: a guide and strategy for their conservation. Oxford (ENG): WWF and IUCN; 1997. p. 355–359.
- Laurencio D, Malone JH. The amphibians and reptiles of Parque Nacional Carara, a transitional herpetofaunal assemblage in Costa Rica. Herpetol Conser Biol. 2009;4:120–131.
- Fox JW. The intermediate disturbance hypothesis should be abandoned. Trends Ecol Evol. 2013;28(2):86–92.
- Rocha LA, Alexio A, Allen G, et al. Specimen collection: an essential tool. Sci. 2014;344(6186):814–815.
