ABSTRACT
Humpback whales perform long migrations from their breeding and nursing areas at low latitudes to feeding grounds at high latitudes. Nonetheless, this strictly dichotomous paradigm of migration is challenged by accumulating examples of occasional or regular feeding at low latitudes for several stocks worldwide. Here, we report multiple evidences of “Stock G” humpback whales feeding in coastal waters, at low latitudes of the Southeast Pacific Ocean. Lunge feeding behavior and defecation were observed in Ecuador, while both lunge- and trap feeding in pursuit of Peruvian anchovy was documented in northern Chile. A photographically re-sighted individual feeding at different latitudes of Chile suggested potential site fidelity to two foraging areas. Whether these feeding behaviours are novel due to changes in prey distribution, intensifying competition from a growing humpback whale population, or simply reflect vastly increased research effort remains unknown. Further research into the feeding ecology of Stock G should help reveal historic and potentially new feeding grounds, prey composition and precise migration paths. Competition from anchovy fisheries, vessel collision and net entanglement are suspected threats.
INTRODUCTION
Humpback whales perform long migrations from high latitude feeding grounds to their breeding and nursing areas in low latitudes [Citation1–3], with the notable exception of the non-migrating Arabian Sea population that remains year-round in the Arabian Sea and Persian Gulf [Citation3,Citation4]. However, the paradigm of this long migration for feeding or breeding strategies is under discussion as an increasing number of studies show humpback whales feeding in mid or low latitudes (from ~5º to ~27º latitude in Northern and Southern Hemispheres), which formerly went unrecognized [Citation5–10]. Some authors highlight the need for whales to find new localities to feed due to increasing population abundance, the potential consequences of climate change on humpback whale prey distribution or the learning of new feeding areas among individuals [Citation10]. However, there is no reason to believe that occasional low-latitude feeding, as documented in this paper, is novel behaviour in the SE Pacific. It may simply have remained unnoticed, partly because whaling had severely depleted populations and partly because before 2000 research effort on humpback whales was scarce in western South America [Citation6].
Humpback whales are generalist predators, which exhibit different foraging strategies related to high primary and secondary productivity areas [Citation11,Citation12]. Feeding strategies depend on the target prey, for example, Alaska humpback whales engage in coordinated lunge-feeding where they feed on euphausiid crustaceans (Euphausia pacifica) and herring (Clupea harengus) [Citation13]. In the NE Atlantic Ocean humpback whales perform lunge feeding and bubble formation in group associations or individuals alone [Citation14]. Off British Columbia (Vancouver) humpback whales trap-feed on small and less concentrated herring schools [Citation15]. Both feeding strategies are used to maintain or increase naturally occurring concentrations of preys [Citation14,Citation15]. The whales set a trap for juvenile fish when they are in small diffuse schools. The fishes are collected near or in the mouth of the whales while hiding from predation by diving birds; then, the whales use their pectoral fins to herd the fish towards their mouths [60]. High fidelity to feeding sites has been demonstrated in the NE and NW Pacific humpback whales by genetic and photo-identification studies [Citation16–18]. Genetic analysis demonstrated finer site fidelity between two different feeding sites spaced 500 km apart in the Russian Pacific [Citation18]. In addition, humpback whales showed different feeding strategies in the two feeding sites, as near Karaginsky Island they foraged on schooling fishes and around the Commander Islands they foraged on euphausiids [Citation19].
In the SE Pacific Ocean the IWC-named breeding Stock G of humpback whales perform long migrations from high latitudes in the Corcovado Gulf (42ºS), Magellan Strait (54ºS) and Antarctica (70ºS) in summer to their breeding and nursing grounds in low latitudes in northern Peru (04ºS), Ecuador, Colombia, Panama and Costa Rica (12ºN) during austral winter [Citation20–26]. Recently, photo identification studies have shown that humpback whales feeding in Antarctic waters breed in northern Peru, Ecuador and Colombia, whereas humpback whales feeding in Magellan Strait breed in Costa Rica and Panama [Citation2,Citation27]. Genetic studies also showed genetic structuring between breeding and feeding areas of the Stock G [Citation28]. However, occasional humpback whale sightings in low latitude upwelling areas during the summer may indicate that not all whales migrate to high latitudes for feeding [Citation6,Citation29]. The Humboldt Current marine ecosystem is recognized for its exceptionally high secondary productivity of small pelagic fishes such as the Peruvian anchovy (Engraulis ringens) due to its year-round high primary productivity upwelling system [Citation29,Citation30]. It extends from 04–07ºS in northern Peru to 37ºS off central Chile. Distinct upwelling cells occur within Chile and one of the most intense and permanent is the Mejillones Peninsula Upwelling System (MPUS at 23ºS) [Citation31,Citation32]. Coastal upwelling is a primary oceanographic process promoting a rich habitat for pelagic organisms, which serve as prey for humpback and other baleen whales [Citation29,Citation30]. While fin whales (Balaenoptera physalus) have repeatedly been observed feeding during austral summer along Mejillones Peninsula (northern Chile), humpback whales were merely documented travelling on their northward or southward migration [Citation32,Citation33].
This study reports the first authenticated cases of humpback whales performing lunge and trap feeding behavior in northern Chile, and presents evidence of lunge feeding and defecation on a key breeding ground in coastal Ecuador.
MATERIAL AND METHODS
Although dedicated line transect surveys for cetaceans were conducted weekly from October 2018 till March 2020 (18 months), all humpback whales in Mejillones Bay (23°1’45”S, 70°29’44”W, ), northern Chile, were initially detected by local fishermen during fishing or ecotourism activities, as part of an opportunistic “citizen science” programme. Alerted researchers (including AMGC) then set out to sea on a 7 m vessel equipped with a 50 HP outboard engine to collect data, photo-identify and monitor the whales for the period they remained in the area. Photo-ID images of the flukes were taken with a CANON T5i Reflex camera and geographic coordinates were recorded with a Garmin Etrex 10 GPS.
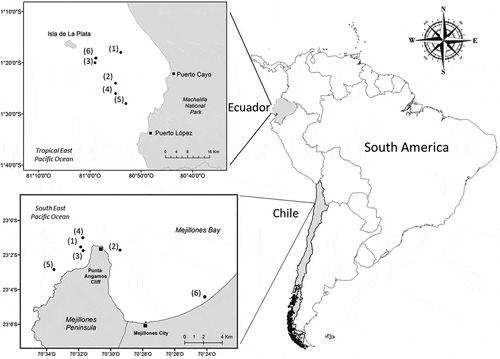
Figure 1. Map of Machalilla National Park in Ecuador and Mejillones Península in northern Chile showing the geographic positions (black dots) of humpback whales performing feeding behavior or defecation. Dates of observations in Ecuador: (1) 21 June 2005 (2) 11 July 2008 (3) 13 August 2008 (4) 15 July 2011 (5) 10 August 2016 (6) 13 August 2017. Dates of observations in Chile: (1) 10 March 2019 (2) 12 March 2019 (3) 16 March 2019 (4) 17 March 2019 (5) 23 March 2019 (6) 18 April 2020
Observations in Ecuador were conducted opportunistically during the humpback whale breeding season (June–October) in the Machalilla Marine Park, in 2005–2017. An 11 m commercial whale-watching vessel was used as platform which sailed daily from Puerto López, Guayas. All observations reported here were made by a marine mammal biologist (C. C.). Defecation is defined as the direct observation at the surface (n = 1) of a normally acting humpback whale suddenly expulsing ventrally a large volume of olive yellow matter, instantly clouding and colouring the otherwise transparent blue water nearest to the body and expanding thereof. Faeces were also identified (n = 3) when in ensuing moments olive yellow faecal matter, some buoyant at the surface and some momentarily sticking to the back or dorsal fin of a whale were clearly observed and photographed. Faeces are sui generis and no credible alternate explanation was available. Blood was never seen.
RESULTS
Northern Chile. Over five days, during their presence from 10 March to 4 April 2019, two adult humpback whales were observed feeding side-by-side on Peruvian anchovy in Mejillones Bay.
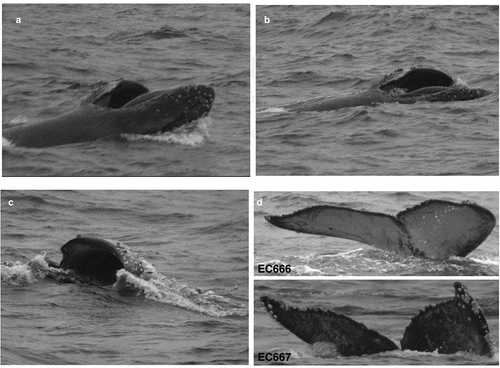
During the observation period anchovies were grouped in big schools of small-sized, juvenile, individuals and both whales performed coordinated lunge feeding and trap feeding strategies (). Lunge feeding consisted of dives of 2 min, followed by the anchovies leaping off the water surface and the whales suddenly breaking the surface simultaneously in close proximity to the center of the school, the mouth wide agape and with distended throat (). Whales rised up to one-third of their body length before falling back to the water. No bubbles were observed, however the emergence of schools of anchovies at the surface one to two seconds before the whales broke the surface suggests that the whales herded the fish schools upward. Observations and photographs of prey at the surface and inside the buccal cavity of the whales and the presence of South American sea lions (Otaria flavescens) and foraging seabirds including Inca terns (Larosterna inca) and gray gulls (Leucophaeus modestus) were registered (). Trap feeding consists in humpback whales remaining close to the surface with open mouth for an extended period of time (minimum 4 s) compared to lunge feeding [Citation15]. Humpback whales were observed performing both lunge and trap feeding in the same observation period. Both whales were preying on schooling immature Peruvian anchovy (Engraulis ringens). When humpback whales started trap feeding, they remained almost stationary at the surface mouths agape for long periods, viz. 12 s and minimum 14 s in two videos (see complementary information) while Inca terns and gray gulls approached the whales’ mouths to prey on anchovies. In the Southern Hemisphere, lunge feeding strategy has been described previously in humpback whales from Magellan Strait but no whales have been observed trap feeding outside of British Columbia [Citation34].
Figure 2. Lunge feeding and trap feeding behaviors by two photo-identified humpback whales as documented off the Mejillones Peninsula, northern Chile. (A) One of the whales observed trap feeding during first day of observation (10 March 2019) with mouth kept open for longer than 4 seconds. Peruvian anchovy at the surface were herded towards its mouth with pectoral fins. (B) The same two humpback whales observed on a second day (12 March 2019) performing trap feeding behavior on schools of Peruvian anchovy. The whales remained in parallel position at the surface with mouths wide agape. (C) Trap feeding by the same two individuals recorded on a third day (16 March 2019). (D) Lunge feeding performed by the same two whales on 17 March 2019. Note anchovies (white arrow) escaping from one whale’s mouth. (E) Lunge feeding observed off Mejillones Peninsula on 17 March 2019. Whales emerged in synchrony, one exposing one third of body while the other engaged in lateral lunge feeding. Seabirds fed on anchovies herded to the surface. (F) Lunge feeding documented for the last time (23 March 2019). White arrows indicate how the whale forcefully expels water through the baleen by closing its mouth. (G) Propeller scars of the bigger humpback whale encountered off Mejillones Peninsula. Note abundant grey gulls hovering above the whale. (H) The individual performing lunge and trap feeding in March 2019 was (I) recaptured and seen lunge feeding in April 2020

One of the humpback whales observed in March 2019 was photographically recaptured in April 2020 in the port area of Mejillones Bay with another humpback whale performing lunge and trap feeding (, I) on Peruvian anchovies. We were able to collect several Peruvian anchovies of size range 8–12 cm with fishing line and small hook, at the exact location and time when humpback whales were seen preying on anchovies. A photo identification analysis of the two humpback whales recorded in Mejillones Bay in March–April 2019 matched one of them with a humpback whale observed feeding on krill (Euphausia sp.) in the Gulf of Corcovado, Chile’s northern Patagonia, during the 2017 austral summer. In addition, the individual showed a propeller scar on its dorsal fin in 2019 but not in 2017 (Paulina Bahamonde and Gustavo Chiang, personal communications to A.M.G.C.).
Coastal Ecuador. On 21 June 2005, two humpback whales were observed lunge feeding in protected coastal waters (at 01°18’S, 80°54’W) of the Machalilla National Park, Ecuador (). The sighting from 10:47 till 11:40 was made opportunistically by one of us (C.C.) from a 11 m fiberglass whale-watching boat equipped with two 115 HP outboard engine, on the Puerto López – Isla de la Plata route. Initially, the whales moved slowly together, changing behavior at 11:21 when both started side lunge feeding. Six times in a row the whales, slowly moving on one side, broke the surface with wide open mouth and extended ventral pleats () before closing mouths while still at the surface. Only one whale lifted its tailstock and flukes above water before diving and considering that the whales did not surface in synchrony, possibly they were diving to different depths. At 11:42 the whales surfaced with mouths shut and resumed their prior behavior of slow travel. No prey species could be seen as, usual at that time of the year, turbidity was very high. The whales photo-identified (by flukes) as EC666 and EC667 in the PWF Ecuadorian catalogue were compared with some 600 individuals from Ecuador, 342 individuals in the catalogue of Fundación Yubarta (Colombia), 36 whales in the Fundación Sentir catalogue (Colombia) and with 65 photos by CEQUA (Chile), but no matches were found. Although feeding had not been observed before, nor since, on other whale-watching excursions in the Machalilla National Park, one local fisherman offered a detailed description of a behavior he observed which can be interpreted as a probable bubble net feeding humpback whale. That occasional feeding off Puerto López may not be so exceptional is suggested by documented observations of locally defecating humpback whales.
Figure 3. (A) Humpback whale lunge feeding in protected coastal waters (01°18S,80°54’W) of Machalilla National Park, Ecuador. (B) Slow-moving whale, tilted 90º on its right side, opens mouth and starts lunge feeding. (C) Whale surfacing with wide open jaws and expanded throat pleats; closing jaws after a few seconds. (D) Flukes photo-ID of feeding humpback whales EC666 and EC667 on 21 June 2005 (Photos: C. Castro – PWF)
Faeces and the actual discharging of faeces were observed on five occasions, as follows. An adult whale EC1373 sighted at 01°24’S, 80°55’W on 11/07/2008 () and adult EC1504 (one of two whales) recorded at 01°21’S, 80°59’W on 13/08/2008 were both photographed with faeces sticking to their dorsum or dorsal fins. Another adult whale was sequentially photographed, surfacing first without, and then with, green-brown faeces sticking to its dorsal fin at 01°26’S, 80°55’W on 15/07/2011.
Figure 4. Evidence of humpback whale defecation at Machalilla National Park, Ecuador: (A) Whale EC1373 at 01°24’S,80°55’W on 11/07/2008 with olive yellow faeces momentarily sticking to dorsal fin. (B) Humpback whale sighted at 01°28’S,80°53’W on 10/08/2016 when suddenly releasing a cloud of faeces of the same olive yellow color (Photos: C. Castro – PWF)

A group of three adult humpback whales was encountered slapping pectoral fins on the sea surface at 01°28’S, 80°53’W on 10/08/2016. After 10 min, the water around one individual suddenly became clouded and discolored by the evident ventral release of a large quantity of olive yellow faeces (). Shrimp boats were fishing in the vicinity (<500 m) and may provide a clue as to a potential prey target.
A potential fifth case occurred on 13/08/2017 very close to the boat at 01°20’S, 80°59’W. However, an attempt to sample the floating olive yellow, apparently faecal matter similar to the confirmed cases, failed ().
Figure 5. Peruvian anchovies (Engraulis ringens) sampled simultaneously when humpback whales were lunge- and trap feeding in Mejillones Bay, northern Chile, in April 2020

Considering that freshly expulsed whale faeces may have positive buoyancy and be sticky (K.V.W., pers. observations in Antarctic waters), the surfacing through a large faecal cloud explain the brief tainting and befouling of parts of the whale’s dorsum and dorsal fin. The speed of digestion and transition of ingested food through a whale’s gastrointestinal tract is rapid [Citation35]. Digestion of a full stomach in baleen whales has been estimated at about 15 hours [Citation36]. The whales observed defecating evidently must have fed locally on the breeding ground.
DISCUSSION
Trap feeding is considered a novel foraging strategy of humpback whales off Vancouver Island, Canada [Citation15] and this is the first record of Stock G whales performing this strategy. It has been suggested that foraging strategies may vary among humpback whale populations depending on their prey type, abundance and geographical area [Citation34]. For example, in the Magellan Strait whales were bubble net feeding on Fuegian sprat (Sprattus fueguensis). In the Antarctic Peninsula, bubble net and lateral lunge feeding were performed to capture Antarctic krill (Euphausia superba), and skimming/lunge-feeding to capture lobster-krill (Munida rugosa) [Citation22]. In western Canada, humpback whales used both trap- and lunge feeding while targeting juvenile Pacific herring (Clupea pallasi) [Citation15,Citation38]. However, the preference of one feeding strategy versus another depending on the type of prey may also be influenced by a variety of factors, such as bottom slope, abundance of prey, individual preferences, prey distribution in the water column and shape of the prey school [Citation13,15,Citation37–41]. McMillan et al. (2018) showed that when herring schools were less dense, humpback whales utilized trap feeding instead of lunge feeding [Citation15]. Trap feeding is a less energetically costly feeding strategy to capture less dense aggregations of schooling fishes [Citation41]. In British Columbia, Canada, the number of humpback whales engaging in trap feeding increased in consecutive years, suggesting that it might be learned from others (horizontal or cultural transmission) [Citation42,Citation43]. Maternal transmission and/or cultural transmission have been observed as calves practice the same feeding behavior used by their mother [Citation11,Citation44]. Whether this foraging strategy has been adopted by Stock G humpback whales from the population in the Northeast Pacific is unknown but considered possible.
In Chile, the recapture of the same whale feeding in the same period (early autumn) in consecutive years suggests feeding site fidelity to Mejillones Bay, possibly on its northward migration to Eastern Tropical Pacific coasts. Humpback whales tend to have higher site fidelity to their feeding grounds than to the breeding grounds in both Northern and Southern Hemispheres [Citation27,Citation45–47]. Site fidelity to the Magallanes Strait feeding ground has been described for Stock G [Citation22,Citation27]. The discovery of new feeding habitats for humpback whales have been attributed to climate change effects presumably modifying prey distribution [Citation48–50] or the expansion of humpback whale populations increasing competition for food compelling whales to find new feeding grounds [Citation10]. However, local fishermen from Mejillones described humpback whales performing lunge feeding behavior before industrial fishing started in the 1980s (Juan Menares, personal communication). The decrease of fish abundance due to industrial fisheries and the severe depletion of humpback whales in the SE Pacific following a century of whaling until 1968 [Citation51,Citation52], might have artificially masked a natural behavior of at least occasional feeding at low latitudes. Moreover, before 2000 dedicated research on SE Pacific humpback whales was minimal [Citation6]. Nonetheless, humpback whales were previously reported feeding at low latitudes in the year-round upwelling system of Peruvian coastal waters [Citation6,Citation29], but no graphical evidence was available. For three days in mid-summer (17–20 February 1996), biologists I. Garcia-Godos and C. Zavala observed two humpback whales from cliffs at San Juan de Marcona (15°20’S) in south-central Peru. The whales, pursued by numerous seabirds, were repeatedly seen feeding at the surface, as well as breaching [Citation6]. One humpback whale sighted in Bahia San Jorge, 200–300 m inshore of southern Antofagasta (23°28.5’S) on 14 March 1987 [Citation53] apparently neither had undertaken a southbound summer migration. We speculate it might have been feeding at the adjacent Mejillones Bay. Humpback whales that oversummer off Chile and Peru are subjected for extended periods to anthropogenic threats, such as vessel collision [Citation33] and fishing gear entanglement [Citation56,Citation58]. For instance, their small-scale distribution at Mejillones Bay has been demonstrated to overlap with the navigation paths of large cargo vessels entering and exiting the highly industrialized and major seaport of Mejillones [Citation33].
CONCLUSION
In conclusion, we here present first graphical evidence of humpback whales occasionally feeding and defecating at two low-latitude coastal locations in the SE Pacific, that is, at Mejillones Peninsula in northern Chile (ca. 23°S) and in waters of the Machalilla National Park, Ecuador (ca. 01°S).
Further research will need to clarify humpback whale migration patterns and feeding ecology in the SE Pacific Ocean, and allow us to understand whether these parameters have been modified significantly by climate-change influence on prey distribution and availability. It is necessary to unravel also how the intense industrial purse-seine fishery of small pelagic fishes in Mejillones Bay (personal observations) is affecting the local abundance of Peruvian anchovy, and generally whether fishing effort is disturbing whale distribution and habitat use in nearby areas of the Humboldt Current System. Moreover, the recent propeller scar observed in one of the humpback whales in Mejillones Bay underlines the evident risk of collision with maritime traffic. The relatively high navigation speed of industrial fishing boats in the bay may endanger the humpback whales and disturb their feeding behavior [Citation33]. The frequent inshore presence of both humpback and fin whales should lead to consideration of additional conservation policies such as a Marine Spatial Planning effort for transiting vessels to avoid the habitual foraging areas. Moreover, a ban on industrial purse-seine fishing for small pelagics inside the bay would greatly favour the conservation of these whales [Citation32,Citation33] but also of small cetaceans such as the dusky dolphin Lagenorhynchus obscurus, common bottlenose dolphin Tursiops truncatus and Burmeister’s porpoise Phocoena spinipinnis, all of which inhabit Mejillones Bay [Citation53,Citation56] where they are thought to commonly prey on anchovies as they do in Peruvian coastal waters [Citation54,Citation55,Citation57].
Acknowledgments
We warmly thank Juan Menares as Director of CIFAMAC NGO and Elias Peñaloza for their contribution with photographs. Juan Menares provided also geographic coordinates of humpback whales sighted during wildlife observation tours in Chile. Paulina Bahamonde and Gustavo Chiang are thanked for comparing our photos of two humpback whales with their own photo-identification catalogue and are credited with finding a match. Logistic support by Ecuadorian tourism operators and personnel of the Machalilla National Park is gratefully acknowledged. Ignacio García-Godos (CEPEC) is thanked for kindly reconfirming an observation of humpback whale feeding at San Juan de Marcona, Ica, Peru. An anonymous reviewer is thanked for providing helpful comments. Logistic support by Ecuadorian tourism operators and Pacific Whale Foundation is gratefully acknowledged.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Clapham PJ. The humpback whale: seasonal feeding and breeding in a baleen whale. In: Mann J, editor. Cetacean societies: field studies of dolphins and whales. Chicago: University of Chicago; 2000. p. 173–196.
- Acevedo J, Aguayo-Lobo A, Allen J, et al. Migratory preferences of humpback whales between feeding and breeding grounds in the Eastern South Pacific. Mar. Mamm. Sci. 2017;33(4):1035–1052.
- Minton G, Collins T, Findlay K,P, et al. Seasonal distribution, abundance, habitat use and population identity of humpback whales in Oman. J Cetac Res Manag. Special Issue on Southern Hemisphere Humpback Whales. 2011;3:185–198. .
- Dakhteh SMH, Ranjbar S, Moazeni M, et al. The Persian Gulf is part of the habitual range of the Arabian Sea Humpback whale population. J Mar Biol Oceanog. 2017;6(3):1–6.
- Gendron D, Urban J. Evidence of feeding by humpback whales (Megaptera novaeangliae) in the Baja California breeding ground, Mexico. Marine Mammal Science. 1993;9(1):76–81.
- Van Waerebeek K, Alfaro-Shigueto J, Arias-Screiber M. Humpback whales off Peru: new records and a rationale for renewed research. Document SC/48/SH1 presented to the IWC Scientific Committee, Aberdeen, 1996. (unpublished), 8pp. Available at http://www.vliz.be/imisdocs/publications/ocrd/243269.pdf
- Alves LCPS, Andriolo A, Zerbini AN, et al. Record of feeding by humpback whales (Megaptera novaeangliae) in tropical waters off Brazil. Mar Mamm Sci. 2009;25(2):416–419.
- Bortolotto GA, Kolesnikovas CKM, Freire AS, et al. Young humpback whale Megaptera novaeangliae feeding in Santa Catarina coastal waters, Southern Brazil, and a ship strike report. Marine Biodiversity Records. 2016;9(1):29–35.
- Findlay KP, Seakamela SM, Meyer MA. Humpback whale “super-groups” – a novel low-latitude feeding behaviour of Southern Hemisphere humpback whales (Megaptera novaeangliae) in the Benguela Upwelling System. PloS ONE. 2017;12(3):1–18.
- Weerdt J, Ramos EA. Feeding of humpback whales (Megaptera novaeangliae) on the Pacific coast of Nicaragua. Mar Mamm Sci. 2019.
- Weinrich MT, Schilling MR, Belt CR. Evidence for acquisition of a novel feeding behaviour: lobtail feeding in humpback whales, Megaptera novaeangliae. Animal Behaviour. 1992;44(6):1059–1072.
- Sharpe F. Social foraging of the southeast Alaskan Humpback whale, Megaptera novaeangliae. Doctoral Thesis, Simon Fraser University, Burnaby, 2001;141 pp.
- Friedlaender AS, Hazen EL, Nowacek DP, et al. Diel changes in humpback whale Megaptera novaeangliae feeding behavior in response to sand lance Ammodytes spp. behavior and distribution. Mar Ecol Progr Ser. 2009;395:91–100.
- Hays HE, Winn HE, Petricig R. Anomalous feeding behavior of a humpback whale. J Mammal. 1985;66(4):819–821.
- McMillan CJ, Towersm JR, Hildering J. The innovation and diffusion of “trap-feeding” a novel humpback whale foraging strategy. Mar Mamm Sci. 2018. DOI:https://doi.org/10.1111/mms.12557
- Baker CS, Steel D, Calambokidis J, et al. Strong maternal fidelity and natal philopatry shape genetic structure in North Pacific humpback whales. Marine Ecology Progress Series. 2013;494:291–306.
- Witteveen BH, Wynne KM. Site fidelity and movement of humpback whales (Megaptera novaeangliae) in the western Gulf of Alaska as revealed by photo-identification. Can J Zool. 2016;1–7.
- Richard G, Titova OV, Fedutin ID, et al. Cultural transmission of fine-scale fidelity to feeding sites may shape humpback whale genetic diversity in Russian Pacific waters. J Heredity. 2018;109(7):1–11.
- Filatova OA, Witteveen BH, Goncharov AA, et al. The diets of humpback whales (Megaptera novaeangliae) on the shelf and oceanic feeding grounds in the western North Pacific inferred from stable isotope analysis. Marine Mammal Science. 2013;29(3):253–265.
- Flores-Gonzalez L. Humpback whales Megaptera novaeangliae in the Gorgona Island, Colombia Pacific breeding waters: population and pod characteristics. Mem Queensl Mus. 1991;30:291–295.
- Scheidat M, Castro C, Denkinger J, et al. A breeding area for humpback whales (Megaptera novaeangliae) off Ecuador. J Cetac Res Manag. 2000;2:165–172.
- Gibbons J, Capella JC, Valladares C. Rediscovery of humpback whale (Megaptera novaeangliae) feeding ground in the Straits of Magellan, Chile. J Cetac Res Manag. 2003;5:203–208.
- Santillán L. Records of humpback whales (Megaptera novaeangliae) in Sechura Bay, Peru, in spring 2009-2010. JMATE. 2008;4(1):29–35.
- Pacheco AS, Silva S, Alcorta B. Winter distribution and group composition of humpback whales (Megaptera novaeangliae) off northern Peru. Latin Am J Aquatic Mammals. 2009;7(1–2):33–38.
- Castro C, Aguayo-Lobo A, Allen J, et al. Humpback whale identification off Ecuador and their migratory connections to Antarctica (Area I And II). Paper SC/64/SH23 presented to the 64th Scientific Committee of the International Whaling Commission. 2013. 5p. Panama City, Panama.
- Hucke-Gaete R, Haro D, Torres-Florez JP, et al. A historical feeding ground for humpback whales in the Eastern South Pacific revisited: the case of northern Patagonia, Chile. Aquatic Conservation: Marine and Freshwater Ecosystems. 2013;23(6):858–867.
- Acevedo J, Rasmussen K, Félix F, et al. Migratory destinations of humpback whales from the Magellan Strait feeding ground, Southeast Pacific. Mar Mamm Sci. 2007;23(2):453–463.
- Félix F, Caballero S, Olavarría C. Genetic diversity and population structure of humpback whales (Megaptera novaeangliae) from Ecuador based on mitochondrial DNA analyses. J Cetac Res Manag. 2012;12:71–77.
- Papastavrou V, Van Waerebeek K. A note on the occurrence of humpback whales (Megaptera novaeangliae) in tropical and subtropical areas: the upwelling link. Forty-Seventh Report of the International Whaling Commission 1997;47:945–947.
- Freón P, Bouchon M, Estrella C, et al. Comparación de los impactos ambientales y aspectos socioeconómicos de las cadenas de producción de anchoveta. Bolet Instit Mar Perú. 2010;25:63–71.
- Escribano R, Marin V, Hidalgo P, et al. Physical-biological interactions in the pelagic ecosystem of the nearshore zone of the northern Humboldt Current System. The oceanography and ecology of the nearshore and bays in Chile. Symposium on linkages and dynamics of coastal systems: open coasts and embayments, Santiago, Chile 2000. Castilla JC, Largier JL (eds) Ediciones Universidad Católica de Chle 2002;p. 145–175.
- Pacheco AS, Villegas VK, Riascos JM, et al. Presence of fin whales (Balaenoptera physalus) in Mejillones Bay, a major seaport area in northern Chile. Revista de biología marina y oceanografía. 2015;50(2):383–391.
- García-Cegarra AM, Pacheco AS. Collision risk areas between fin and humpback whales with large cargo vessels in Mejillones Bay (23°S), northern Chile. Marine Policy. 2019;103: 182–186.
- Acevedo J, Plana J, Aguayo-Lobo A, et al. Surface feeding behavior of humpback whales in the Magellan Strait. Revista de biología marina y oceanografía. 2011;46(3):483–490.
- Ridgway SH. Mammals of the Sea – biology and Medicine. Springfield, Illinois: C.C. Thomas; 1972.
- Kawamura A. Influence of chasing time to stomach contents of baleen and sperm whales. Scient Rep Whale Res Instit. 1971;23:27–36.
- McMillan CJ. How important are herring to humpback whales? The role of herring in meeting the energetic requirements of humpback whales in a British Columbia feeding ground. M.Sc. dissertation, Simon Fraser University, Burnaby, Canada, 2014;68 pp.
- Jurasz CM, Jurasz VP. Feeding modes of the humpback whale (Megaptera novaeangliae) in Southeast Alaska. Scient Rep Whale Res Inst Tokyo. 1979;31:69–83.
- Hazen EL, Friedlaender AS, Thompson MA, et al. Fine-scale prey aggregations and foraging ecology of humpback whales Megaptera novaeangliae. Mar Ecol Progr Ser. 2009;395:75–89.
- Goldbogen JA, Calambokidis J, Croll DA, et al. Foraging behavior of humpback whales: kinematic and respiratory patterns suggest a high cost for a lunge. J Experim Biol. 2008;211:3712.3719.
- Allen J, Weinrichn M, Hoppitt W, et al. Network-based diffusion analysis reveals cultural transmission of lobtail feeding in humpback whales. Science. 340(6131):485–488
- Noad MJ, Cato DH, Bryden MM, et al. Cultural revolution in whale songs. Nature. 2000;2013(408):537.
- Stamation KA, Croft DB, Shaughnessy PD, et al. Observations of humpback whales (Megaptera novaeangliae) feeding during their southward migration along the coast of southeastern New South Wales, Australia: identification of a possible supplemental feeding ground. Aquat Mamm. 2007;33:165–174.
- Calambokidis J, Steiger GH, Evenson JR, et al. Interchange and isolation of humpback whales off California and other North Pacific feeding grounds. Mar Mamm Sci. 1996;12:215–226.
- Calambokidis J, Steiger GH, Straley J. Movement and population structure of humpback whales in the North Pacific. Mar Mamm Sci. 2001;17:769–794.
- White GC, Burnham KP. Program MARK: survival rate estimation from both live and dead encounters. Bird Stud. 1999;46(Supplement):S120–139.
- Perry AL, Low PJ, Ellis R, et al. Climate change and distribution shifts in marine fishes. Science. 2005;308:1912–1925.
- Simmonds MP, Isaac SJ. The impacts of climate change on marine mammals: early signs of significant problems. Oryx. 2007;41:19–26.
- Askin N, Belanger M, Wittnich C. Humpback whale expansion and climate-change evidence of foraging into new habitats. J Mar Anim Ecol. 2008;9(1):13–17.
- Van Beneden PJ. Histoire Naturelle de la baleine à bosse (Megaptera boops). Mem Cour Acad R Belg. 1887;40:1–42.
- Clarke RW. Catches of sperm whales and whalebone whales in the Southeast Pacific between 1908 and 1975. Rep Int Whal Commn. 1980;30:285–288.
- Guerra C, Van Waerebeek K, Portflitt G, et al. Presencia de cetáceos frente a la segunda region de Chile. The presence of cetaceans off Northern Chilean coast. Estud Oceanol. 1987 ;6:87–96.
- Van Waerebeek K, Reyes JC, Read AJ, et al. Preliminary observations of bottlenose dolphins from the Pacific coast of South America. In: Leatherwood S, Reeves RR, editors. The Bottlenose Dolphin. San Diego: Academic Press; 1990. p. 653.
- Reyes JC, Van Waerebeek K. Aspects of the Biology of Burmeister’s porpoise from Peru. Report Int Whaling Com. 1995;16:349–364.
- García-Cegarra AM. Interacciones positivas y negativas entre ser humano y cetáceos en el Pacífico Sureste. Antofagasta (Chile): Universidad de Antofagasta, 2019. Availabe online at: http://repositorio.conicyt.cl/handle/10533/236381
- García-Godos I, Van Waerebeek K, Reyes JC. et al. Prey occurrence in the stomach contents of four small cetacean species in Peru. Lat Amer J Aquat Mamm. 2007;6(2):171–183.
- García-Godos I, Van Waerebeek K, Alfaro-Shigueto J, et al. Entanglements of large cetaceans in Peru: few records but high risk. Pac Sci. 2013;67(4):523–532.
- Kosma MM, Werth AJ, Szabo AR, et al. Pectoral herding: an innovative tactic for humpback whale foraging. R Soc Open Sci. 2019;6:191104.
