ABSTRACT
In the Neotropics, Panopinae spider flies are the most diverse within Acroceridae, specializing as endoparasites of mygalomorph spiders. Camposella insignata Cole, 1919 is one of the most enigmatic flies within this subfamily, given its conspicuously enlarged antennal flagella and apparent rarity, with only two specimens known to this day. This paper describes recently collected C. insignata specimens from the Ecuadorian Andes and provides pictures and measurements. We also discuss the possible function of the enlarged antennal flagella and give recommendations on future collection efforts to increase knowledge about this enigmatic genus.
Acrocerids are cosmopolitan flies whose planidia develop as spider endoparasites [Citation1–3]. Adults have a small head, swollen abdomen, hunchbacked thorax, and in most species, elongated mouthparts [Citation4]. They diversified during the Cretaceous [Citation5,Citation6] and their monophyly is supported based on morphological characters, endoparasitic lifestyle [Citation7–9], and molecular evidence [Citation6]. There are approximately 530 species [Citation10] in five subfamilies: Acrocerinae, Cyrtinae, Ogcodinae, Panopinae, and Philopotinae [Citation11,Citation12].
In the Neotropics, there are 19 genera and 100 species. Recently, taxonomic studies [Citation13–15], species descriptions [Citation10,Citation16,Citation17], and generic revisions [Citation18] have increased the interest in this group. Panopinae are parasitoids of mygalomorph spiders [Citation2,Citation6,Citation16]. They can be diagnosed by a cylindrical or paddle-shaped flagellum that lacks an arista, widely separated postpronotal lobes, presence of tibial spines, and wings with cells m3, d, bm, and basal r4+5 present [Citation9,Citation19]. In the Neotropics, Panopinae is the most species-rich subfamily with ten genera, six which are endemic: Archipialea, Camposella, Coquena, Exetasis, Lasioides, Pialea, and Pteropexus. Five Panopinae species have been reported for Ecuador: Camposella insignata, Lasia ecuadorensis, Ocnaea falcifer, O. gigas and Pialea ecuadorensis.
Morphologically, Camposella is related to Eulonchus and Lasia [Citation11]. Its only species, C. insignata can be identified by its conspicuous enlarged antennal flagella modified into paddle-like plates [Citation11,Citation20,Citation21]. The holotype was sent to the National Museum of Natural History (USNM) by Francisco Campos-Rivadeneira [Citation22–24], and described by Frank Cole (1919) [Citation20,Citation25]. Later, another specimen was reported from the National Museum of Natural History (MNHN) [Citation21]. No other records have been reported ever since. Here, we describe a recently collected C. insignata specimen, report the finding of two museum specimens, provide pictures of its habitat, and a preliminary list of potential hosts.
We follow the terminology by Schlinger et al. [Citation10], references therein [Citation4,Citation9,Citation26,Citation27] and, Kirk-Spriggs & Sinclair (eds.) for wing venation [Citation28]. Specimens were identified following Schlinger [Citation10], and Cole [Citation20]. Coloration is described after Köhler’s Catalogue [Citation29]. Photographs were taken using an Olympus DP73 camera adapted to an Olympus SZX16 stereo microscope. To obtain images with greater focus depth, we used the Z-Stack function in cellSens Dimensions v1.8.1 © (Olympus Corp. 2009–2013). Examined specimens are deposited at Museo de Zoología, Universidad San Francisco de Quito (ZSFQ) and Museo de Zoología, Pontificia Universidad Católica del Ecuador (QCAZ), Quito, Ecuador.
The following measurements were taken from ZSFQ-i5155 specimen (see Distribution): head capsule length (HCL), in frontal view, length from the dorsal occipital border to inferior head border; head capsule width (HCW), length across head’s widest section (eyes included); third antennal segment length (TAS), in lateral view, length from middle of antenna base to distal tip; antennal length (ANL), length from middle of first antennal segment base to antenna’s most distal tip; antennal width (ANW), length across the broadest section of the antenna; proboscis length (PL), in ventral view, length from proboscis base to its most distal tip; thorax length (TL), in dorsal view, length from pronotum anterior border to posterior border of scutellum; abdomen length (AL), length from first abdominal segment’s anterior border to posterior border of the last abdominal segment (excluding genitalia); and abdomen width (AW), length across the broadest part of the abdomen. All measurements were taken five times, and the mean (n = 5) and standard deviation are reported.
Camposella insignata Cole, 191
Diagnosis: 1) antennae contiguous, visible dorsally, and separated by a medial ridge, 2) second antennomere trapezoid-shaped and tapering towards the apex, 3) second antennomere with light chrome-orange short setae, 4) proboscis olive-brown, lighter towards the tip, 5) and covered with short sulfur-yellow setae, 6) posterior half of scutum with disperse pilosity, not extending anteriorly, 7) abdominal tergites 1–4 with short setae, 8) third medial cell (m3), discal cell (d), basal medial cell (bm), and r4+5 cell present, 9) cell r4+5 narrow and elongated, 10) first branch of medial cell (M1) not in contact with wing’s lower margin, and connected with the lower large branch of cell r4+5, 11) bm not directly in contact with m3, but connected through a short vein. Head. Capsule and eyes dusky-brown, eyes covered with proclinate dark grayish brown pilosity. Orbital setae behind eyes dark grayish brown, posterior setae immediately behind and extending towards occipital border longer, proclinate and light chrome-orange. Inner and outer occipital margins visible in frontal view, meeting below head capsule in a pointed plate with numerous dark grayish brown long setae. Eyes contiguous above antennal insertions, separated at about the upper third section and widely separated below antennae. Antennae contiguous at the base, separated by a medial ridge. First antennomere devoid of setae and visible, trapezoidal in shape, base dusky-brown turning walnut brown towards the apex. Plate above antennal base, and between eyes, similar to the first segment. Second segment about a third longer than the first, trapezoidal and tapering towards the distal section, dusky-brown with short light-chrome orange setae, originating from midline and reaching distal border. Third segment as broad as the second at its base, tapering to form a thin plate of uniform width throughout all its length, when viewed laterally, it forms a paddle-like plate about twice as long as head capsule, and its width less than half its length, fuscous-colored and devoid of setae. Proboscis rudimentary, slender, and pointy; olive-brown and lighter towards the tip, covered with short sulphur-yellow setae. Region below antennae concave. Occiput slightly swollen. Ocellar triangle rudimentary, same color as head capsule, with a small central concavity with dark grayish brown setae; ocelli absent. Thorax. Dorsally, large and swollen with a rounded profile. About two-thirds of anterior half of scutum covered with abundant medium chrome-orange pilosity that, when looked at from certain angles, switches to light chrome-orange. Posterior half of scutum with disperse dark grayish brown pilosity. Thorax integument vandyke-brown, as postalar callus, which is also covered with dark grayish brown setae. Scutellum flat and sub-rectangular; coloration and pilosity similar to scutum, except for lateral and posterior margins that have lighter setae. Postpronotal lobes swollen and covered with similar pilosity as that covering the scutum; laterally, triangular-shaped and with dense pilosity at the upper margin, and with a dense group of setae at the lower corner. Upper pleura swollen, with maroon integument, medium chrome-orange pilosity anterodorsally, and dusky-brown elsewhere. Squamae smoky-white at the base, with long dusky-brown setae turning hyaline towards the posterior region, and short dispersed setae over its surface. Inferior margin of squamae with long dusky-brown setae and posterior margin with short ones. Legs. Coxae uniformly maroon with light orange-yellow setae more abundant towards lateral and ventral margins. Femora uniformly maroon, anteriorly with long reclinate light orange-yellow setae. Medium and hind femora with long reclinate dusky-brown setae. Tibiae maroon at its base and becoming lighter towards distal portion; distal portion of the tibia wider, particularly at hind leg. Tibiae with pointed apical spur on fore and medium legs, blunter on hind legs, reclinate dusky-brown setae present on tibiae but shorter and sparser from fore to hind tibiae, which only has a few short setae. Tarsi cream-yellow with simple claws and no empodia or pulvilli. Wings: Brownish-hyaline, darker towards the base, without markings or microtrichia. Veins sepia colored. Third medial cell (m3), discal cell (d), basal medial cell (bm), and cell r4+5 present. M1 not contacting the wing’s lower margin, continuously connected to the lower large branch of cell r4+5. Cell bm not directly in contact with m3, but connected through a short vein. R4 not straight as in holotype but wavy, with proximal half pointing towards wing’s dorsum, and distal half towards ventral margin. Abdomen. Tergites one to four vandyke-brown, rounded and broader than thorax, covered with dusky-brown short setae, except for medial zone in which setae are sparse. Viewed laterally, abdomen curved. Tergite five covered with long light-chrome orange setae. Sternum concave and covered with light-chrome orange pilosity, except for median line that is devoid of setae, and laterally where setae are dusky-brown. Genitalia: Viewed laterally, epandrium dusky-brown with upper side concave and lower side convex, converging on a pointed apex. Gonocoxite cuticle raw-umber and covered in vandyke-brown, and light chrome-orange setae. Upper and lower margin parallel, and posterior margin convex. Gonostylus hyaline concave, darker at the tip. Phallus dusky-brown thin and, dorsally, ending in two divergent tips.
Measurements (in milimeters): HCL = 2.88 ± 0.04; HCW = 2.94 ± 0.1; TAS = 5.69 ± 0.02; ANL = 5.93 ± 0.02; ANW = 2.50 ± 0.01; PL = 4.22 ± 0.02; TL = 7.63 ± 0.24; AL = 8.82 ± 0.07; AW = 8.98 ± 0.06.
Distribution: C. insignata is known from five localities in the northern Ecuadorian Andes. Although the type locality was not mentioned in the original description, the name “Aloag Ecua.” is written on its label. Aloag is a small town on an inter-Andean valley, ca. 20 km SSW of Quito, at 2922 m.a.s.l [Citation30,Citation31]. Campos (1960) [Citation32], however, cites “Guayaquil” as the collecting locality for the holotype. Guayaquil is a city on the Pacific coastal lowlands. Since all localities of C. insignata are in the Andes, we consider that Aloag is the correct type locality, and the citation of Guayaquil was probably an error. Of all known localities, Aloag is the only one above 2000 m.a.s.l. Aloag was probably used as a general name for the collecting area, but the holotype was probably collected at lower elevations, in areas easily reachable by road ca. 20 km W of Aloag. Specimen ZSFQ-i5155 was collected at Mindo Scientific Station (−0.0379°, −78.7616°; 1460 m.a.s.l.), province of Pichincha, on 21 October 2017. The station is managed by Universidad San Francisco de Quito and is located near Mindo. This is a small town on the northwestern slopes of the Andes, embedded on a matrix of agricultural lands, secondary and primary forest. The specimen was collected on a trail crossing secondary forest and leading to a primary forest plot (). Specimen QCAZ I 259303 was collected at El Pahuma Reserve (0.02586°, −78.6324°; 1941 m.a.s.l.), province of Pichincha, on 29 January 2012, and QCAZ I 259302 at Otonga Reserve (ca. −0.40°, −78.00°; 2000 m.a.s.l.), province of Cotopaxi, on 23 January 1994. All three localities are on the Western Andean Evergreen Mountain Forest ecosystem [Citation33]. The specimen at the MNHN of Paris was reported only as “Quito”, but it was probably collected at one of the localities on the northwestern Andean slopes (Mindo, Gualea, Pacto-Pachijal) visited by Capitan Fernand Dard D’Espinay, French military attaché in Venezuela and Ecuador, who collected natural history specimens in Ecuador between 1921–1922 [Citation34–38].
Material examined: Male. “ECUADOR COTOPAXI”, “OTONGA 2000 m”, “23JAN1994 GOnore”, “QCAZ I”, “259,302” (QCAZ); Male. “Ecuador Pichincha”, “El Pahuma 1941 m”, “0.0258638 − 78.63236”, “29Ene.12 A.Péres”, “QCAZ I”, “259,303” (QCAZ); Male. “Ecuador Pichincha”, “Mindo 1460 m”, “Lat: −0.037892°; Lon: −78.761611°”, “S. Ramos 21Oct2017”, “SR061”, “Net collecting/ Bushes next to road/ Secondary forest” (, collection site), “ZSFQ-i5155” (ZSFQ) ().
Figure 2. Photographs of the C. insignata ZSFQ-i5155 specimen. Lateral (a), frontal (b), and dorsal (c). Scale bar: 1 mm. Photo credits: AGTR

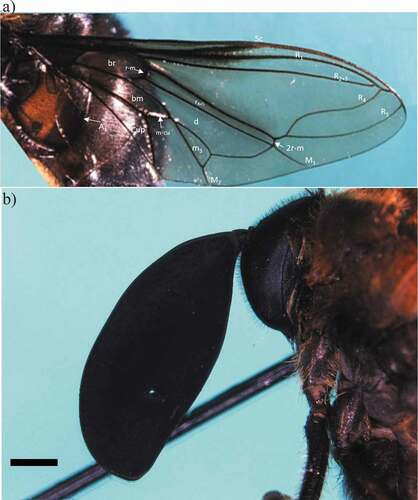
Figure 3. Photographs of the C. insignata ZSFQ-i5155 specimen. Wing (a) and head in lateral view showing the antenna (b). Scale bar: 1 mm. Abbreviations: A1, first branch of anal vein; bm, basal medial cell; br, basal radial cell; Cup, posterior cubital cell; d, discal cell; M1, first branch of medial vein; M2, second branch of medial vein; m-cu, medial cubital crossvein; m3, third medial cell; R1, anterior branch of radius; R2+3, second branch of radius; R4, upper branch of third branch of radius; R5, lower branch of third branch of radius; r4+5, r4+5 cell; r-m, radial-medial crossvein; 2 r-m, second radial-medial crossvein; Sc, subcostal vein. Photo credits: AGTR
Other known specimens: Holotype: Male. “Aloag Ecua.”, “F Campos R”, “1919” [Citation20,Citation25] (USNM); Male. “MUSEUM PARIS”, “ÉQUATEUR”, “Quito?”, “Cap D’ESPINAY 1922” [Citation21,Citation39]. “Camposella”, “insignata, “Cole”, “Det. E Brunetti 1924”, “Camposella”, “insignata”, “Cole”, “Brunetti det. 1924” (MNHN).
Comments: Specimen ZSFQ-i5155 antennae were accidentally broken after pictures and measurements were taken. One was glued back, and the other is kept on a tube beside the specimen. Specimen QCAZ I 259302 was missing both antennae.
Remarks
Cole [Citation20] suggested that the enlarged antennal flagella of this species might be a product of sexual selection; antennae would be antler-like structures to indicate male fitness [Citation40]. There are several examples of sexually selected characters in flies; eye-stalks, for example, have appeared independently on several families [Citation41]. In situ studies of the reproductive fitness of males with varying flagella sizes would help test this hypothesis.
Enlarged flagella might also help males locate females [Citation20] since a larger surface would accommodate more sensilla [Citation42]. Given the sexual dimorphism seen in related genera, it is likely that females have smaller flagella [Citation10]. Even if not as large, enlarged flagella might help females locate spider hosts or oviposition sites [Citation2]. Studies on antennal morphology and ultrastructure would help to understand their role in olfaction.
There are at least nineteen mygalomorph species () that might serve as hosts for Panopinae flies on the northwestern Andean slopes of Ecuador, where C. insignata inhabits. Unfortunately, information on Ecuadorian mygalomorphs is scarce and many species remain undescribed in this part of the Tropical Andes biodiversity hotspot [Citation43]. Further field studies in this region might reveal more Panopinae species. Given the apparent rarity of these flies [Citation2], a possible strategy could be to rear parasitized spider hosts until adults emerge [Citation16].
Table 1. Mygalomorph spider species found on the Andean slopes of northwestern Ecuador, where C. insignata inhabits, and that might serve as hosts for Panopinae spider flies
Author contributions
AGTR first detected the ZSFQ-i5155 specimen at ZSFQ Collection, identified the specimens, took the measurements and photographs, and wrote the manuscript. DFCH collected and provided information on the possible hosts of the species, analyzed the localities and distribution, and reviewed the manuscript. SARR collected the specimen from Mindo. GMRC coordinated the description process, examined specimens of C. insignata at QCAZ and ZSFQ, collaborated on the morphological description, wrote the manuscript, and assembled the table and figures.
Acknowledgments
We would like to thank Dr J. Gillung, who pointed us to genus identification and facilitated literature on the topic. V. Crespo-Pérez and F. Salazar kindly loaned the C. insignata specimen from QCAZ Collection. Dr S. Winterton kindly provided information on the holotype and the specimen at the Paris Museum. Comments from the editor, two anonymous reviewers, and Giovanni Onore helped to improve the quality of the manuscript. Universidad San Francisco de Quito USFQ supported this study through funds granted to Instituto de Biodiversidad Tropical iBIOTROP and Colegio de Ciencias Biológicas y Ambientales COCIBA. The C. insignata specimen ZSFQ-i5155 was collected under investigation permit No. 018-2017-IC-FAU-DNB/MAE issued by Ministerio del Ambiente del Ecuador.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Nartshuk EP. Family Acroceridae. Contributions to a manual of Palaearctic Diptera (with special References to flies of economic importance). In: Papp L, Darvas B editors. Nematocera and Lower Brachycera. Budapest: Science Herald; 1997. Volume 2. 469–485.
- Schlinger EI. The biology of Acroceridae (Diptera): true endoparasitoids of spiders. In: Nentwig W, Berlin H editors. Ecophysiology of Spiders. New York London& Tokyo (Paris): Springer Verlag; 1987.448.
- Gillung JP, Borkent CJ. Death comes on two wings: a review of dipteran natural enemies of arachnids. J. Arachnol. 2017;45(1):1–19.
- Schlinger Evert I. Acroceridae. Manual of Nearctic Diptera. In: McAlpine JF, Peterson BV, and Shewell GEeditors. Monograph. Ottawa: Agriculture Canada Research Branch; 1981. p. 575–584.
- Gillung JP, Winterton SL. A review of fossil spider flies (Diptera: Acroceridae) with descriptions of new genera and species from Baltic Amber. J. Syst. Palaeontol. 2018;16(4):325–350.
- Winterton SL, Wiegmann BM, Schlinger EI, et.al. Phylogeny and Bayesian divergence time estimations of small-headed flies (Diptera: acroceridae) using multiple molecular markers. Mol. Phylogenet. Evol. 2007;43(3):808–832.
- Woodley NE. Phylogeny and classification of the Orthorrhaphous Brachycera. Manual of Nearctic Diptera. In: McAlpine JF, editor. Monograph. Ottawa: Agriculture Canada Research Branch; 1989. p. 1371–1395.
- Yeates DK. Relationships of extant lower Brachycera (Diptera): a quantitative synthesis of morphological characters. Zool Scr. 2002;31(1):105–121.
- Winterton S. Review of Australasian spider flies (Diptera, Acroceridae) with a revision of Panops Lamarck. Zookeys. 2012;172:7–75.
- Schlinger EI, Gillung JP, and Borkent CJ, et.al. New spider flies from the neotropical region (Diptera, Acroceridae) with a key to New World genera. Zookeys. 2013;270:59–93.
- Gillung JP, and Winterton SL. Evolution of fossil and living spider flies based on morphological and molecular data (Diptera, Acroceridae). Syst Entomol. 2019;44(4):820–841.
- Gillung JP, Winterton SL, Bayless KM, et al. Anchored phylogenomics unravels the evolution of spider flies (Diptera, Acroceridae) and reveals discordance between nucleotides and amino acids. Mol Phylogenet Evol. 2018;128:233–245.
- Gillung JP, de Carvalho CJB. Acroceridae (Diptera): a pictorial key and diagnosis of the Brazilian genera. Zootaxa. 2009;2175(1):29–41.
- González CR, Elgueta M, Ramirez F, et.al. A catalog of Acroceridae (Diptera) from Chile. Zootaxa. 2018;4374(3):427–440.
- Gillung JP, Almeida JC, and Rodrigues PFM, et al. Checklist of Acroceridae, Mydidae and Therevidae (Diptera) from Mato Grosso do Sul, Brazil. Iheringia Série Zool. 2017;107.
- Barneche JA, Gillung JP, González A, et.al. Description and host interactions of a new species of Exetasis Walker (Diptera: acroceridae), with a key to species of the genus. Zootaxa. 2013;3664(4):525–536.
- Barahona-Segovia RM, Guzmán VV, Barceló M, et al. A new spider fly (Diptera: acroceridae: ogcodinae: ogcodes Latreille) from Chiloé Island’s evergreen forest and new distributional records for other spider flies in Chile. Zootaxa [Internet]. 2020 [cited 2021 Mar 30];4779:51–64. https://europepmc.org/article/med/33055798.
- Gillung JP, Nihei SS. Evolution of Philopotinae, with a revision and phylogeny of the New World spider fly genus Philopota Wiedemann (Diptera, Acroceridae). Zool J Linn Soc. 2016;176(4):707–780.
- Borkent CJ, Gillung JP, Winterton SL. Jewelled spider flies of North America: a revision and phylogeny of Eulonchus Gerstaecker (Diptera, Acroceridae). Zookeys. 2016;619:103–146.
- Cole FR. A new genus in the Dipterous Family Cyrtidae from South America. Entomol News Proc Entomol Sect. 1919;30:271–274.
- Brunetti E. LXIX. –New and little-known Cyrtidæ (Diptera). Ann Mag Nat Hist. 1926;18(107):561–606.
- Ramón G, Jarrín P, Pérez Pimental R, et.al. Francisco Campos-Rivadeneira and Roberto Levi-Castillo: their lives and contributions to the study of mosquitoes (Diptera: culicidae) in Ecuador. Biomédica. 2019;39:172–198.
- Barragán AR, Dangles O, Cardenas RE, et al. The history of entomology in Ecuador. Ann la Société Entomol Fr. 2009;45(4):410–423.
- Mauffray WF, Tennessen KJ. A catalogueand historical study of the Odonata of Ecuador. Zootaxa. 2019;4628(1):1–265.
- NMNH. 2020. Camposella insignata Cole: acroceridae: diptera: insecta: arthropoda. Dep. Entomol. Collect. Database.
- Cumming JM, and Wood M . Adult morphology and terminology. In: Brown BV, Borkent A, and Cumming JMeditors. Manual of Central American Diptera. Ottawa: NRC Research Press; 2009. p. 9–50.
- Gillung J, Winterton S. New genera of philopotine spider flies (Diptera, Acroceridae) with a key to living and fossil genera. Zookeys. 2011;127:15–27.
- Kirk-Spriggs AH, Sinclair BJ, editors. Manual of Afrotropical Diptera. Nematocerous Diptera and lower Brachycera. 2. Pretoria: Suricata 5. South African National Biodiversity Institute; 2017.
- Köhler G. Color catalogue for field biologists. Offenbach (Germany): Herpeton; 2012.
- Sabrosky C. A synopsis of the Larvaevorid flies of the genus Eudejeania. Proc United States Natl Museum. 1947;97(3215):141–156.
- Brown FM. A gazetteer of entomological stations in Ecuador. Ann Entomol Soc Am. 1941;34(4):809–851.
- Campos FR. Las moscas (Brachycera) del Ecuador. Rev Ecuat Hig Med Trop. 1960;17:1–66.
- Ministerio del Ambiente. Sistema De Clasificación De Ecosistemas De Ecuador Continental. Quito: Subsecretaría de Patrimonio Natural; 2013.
- D’Espinay F. Quelques observations sur les tremblements de terre des 5 et 6 avril 1922 à Quito et dans la région avoisinante. La Géographie Bull la Soc Géographie. 1923;34:210–214.
- Anonymous. Actes administratifs. 214a. Réunión des Naturalistes du Muséum. Bull du Muséum Natl d’Histoire Nat. 1923;29:1.
- Anonymous. Membres de la Société des Américanistes. J la Société des Américanistes Paris. 1922;14:ix–xxiii.
- Le Cerf F. Descriptions de formes nouvelles de Lépidoptéres rhopalocères. Bull du Muséum Natl d’Histoire Nat. 1923;19:360–367.
- Berlioz J. Étude de la Collection de Trochilidés rapportée par Mle Capitaine d’Espinay de la región de Quito (Équateur). Bull du Muséum Natl d’Histoire Nat. 1924;30:171–177.
- MNHN. Acroceridae [Internet]. 2020 [cited 2020 Jul 15]. Available from: https://science.mnhn.fr/institution/mnhn/list?full_text=Acroceridae.
- Wilkinson GS, and Dodson GN. Function and evolution of antlers and eye stalks in flies Choe, J, and Crespi , B The Evolution of Mating Systems in Insects and Arachnids. Cambridge (UK): Cambridge University Press 1997 310–328.
- Warren I, Smith H. Stalk‐eyed flies (Diopsidae): modelling the evolution and development of an exaggerated sexual trait. Bioessays. 2007;29(3):300–307.
- Wicker-Thomas C. Pheromonal communication involved in courtship behavior in Diptera. J Insect Physiol. 2007;53(11):1089–1100.
- Mittermeier RA, Robles-Gil P, Hoffman M, et al. Hotspots revisited. Mexico: CEMEX; 2004.
- Araignées BL. Mission du Service géographique de l’armée pour la mesure d’un arc du méridien équatorial en Amérique du Sud (1899-1906). Paris: Gauthier-Villars, Imprimeur Libraire du Bureau des Longitudes, de l'Ecole Polytechnique; 1913. p. 78–119.
- Coyle F. A revision of the funnelweb mygalomorph spider subfamily Ischnothelinae (Araneae, Dipluridae). Bulletin of the AMNH. Bull Am Museum Nat Hist. 1995;226:133.
- Drolshagen B, Bäckstam CM. A taxonomic review of the mygalomorph spider genus Linothele Karsch, 1879 (Araneae, Dipluridae). Zoosystema. 2021;43(10):163–196.
- Dupérré N, Tapia E. Descriptions of four kleptoparasitic spiders of the genusMysmenopsis(Araneae, Mysmenidae) and their potential host spider species in the genus Linothele (Araneae, Dipluridae) from Ecuador. Zootaxa. 2015;3972(3):343–368.
- Dupérré N, Tapia E, Quandt D, et al. From the lowlands to the highlands of Ecuador, a study of the genus masteria (araneae, mygalomorphae, dipluridae) with description of seven new species. Zootaxa. 2021;5005(4):538–568.
- Simon E. Révision des Aviculariidae de la République de l’Ecuador. Actes la Société Linnéenne Bordeaux. 1889;42:399–404.
- Dupérré N, and Tapia E On the putatively incorrect identification and “redescription” of Paratropis elicioi Dupérré 2015 (Paratropididae, Araneae) with the description of two new sympatric species from Ecuador . 2020;4869(3): 326–346.

