ABSTRACT
We investigated the role of environmental and temporal variables on the activity of 20 species of aerial insectivorous bats at a site in tropical premontane forest in Monteverde, Costa Rica. The primary study site was an opening in secondary forest adjacent to the Children’s Eternal Rainforest. We passively monitored the echolocation calls of all bats detected by an Anabat II detector all night for 1,147 nights from November 2000 through August 2010, from which we were able to identify the species in more than 250,000 bat passes. Simultaneously, we recorded environmental variables. Five species accounted for most of the activity, but the relative frequency of these species fluctuated widely over the ten-year period. The likelihood of any one of the three most common species being present was significantly influenced by wind, rain, phase of the moon, the time of night, the season of the year, and the presence of the other two species. In general, strong winds are associated with an increase in bat activity, moderate to heavy rain seems to halt bat activity, full moon appears to depress bat activity, and bats seem to be most active early in the evening. The number of bat passes at the study site declined over the ten-year period, possibly at least partially due to bat preference for foraging near bright lights, which increased markedly during the period due to development for tourism.
RESUMEN
Investigamos el papel de las variables ambientales y temporales de la actividad de 20 especies de murciélagos insectívoros aéreos en un sitio de bosque premontano tropical en Monteverde, Costa Rica. El sitio de estudio era un claro en el bosque secundario adyacente Al Bosque Eterno de los Niños. Por 1,147 noches, a partir de noviembre de 2000 hasta agosto de 2010, monitoreamos durante toda la noche las llamadas de ecolocalización de todos los murciélagos detectados por el grabador Anabat II, de las cuales hemos podido identificar la especie en más de 250.000 pases de murciélago. Al mismo tiempo, registramos las variables ambientales. Cinco especies representan la mayor parte de la actividad, pero la frecuencia relativa de estas especies fluctuó considerablemente durante los diez años del período. Es probable que cualquiera de las tres especies más comunes fuera significativamente influenciada por el viento, la lluvia, la fase de la luna, la hora de la noche, la estación del año, y la presencia de las otras dos especies. En general, los vientos fuertes están asociados con un aumento en la actividad de murciélagos, las lluvias fuertes a moderadas parecen detener la actividad de los murciélagos, la luna llena parece disminuir la actividad de los murciélagos, y los murciélagos parecen ser más activos al inicio de la noche. El número de pases de murciélago en el lugar de estudio disminuyó durante los diez años del período, posiblemente debido a un gran incremento en luces brillantes por los negocios de turismo.
Introduction
By acoustically monitoring the echolocation calls of bats, it has become possible to investigate activity and habitat use over time and space [Citation1]. Even though bats, being nocturnal, are notoriously difficult to observe, ultrasonic recording devices and related software are now widely available and used by thousands of bat biologists. These devices enable the user to monitor bat calls continuously during the night. These recordings enable the user, and to an increasing extent, computer algorithms, to identify the species of bats recorded. Vast amounts of data can thereby be generated [Citation1]. To date, however, there have been few long-term studies based on such data, e.g [Citation2].
Clearly, activity patterns of bats will be affected by many variables, including temperature, light, wind, moon phase, vegetation, time of night, insect abundance, etc., as was demonstrated in an extensive investigation of tropical bats in northern Australia [Citation3]. The extent to which these biotic and physical variables affect bat activity must be taken into account [Citation4–6]. Synchronization of insectivorous bat activity with insect activity is crucial [Citation7,Citation8], and bats must cope with any physical variables that might affect foraging success, including moonlight [Citation9–11], wind [Citation12], rainfall [Citation13], and temperature [Citation5]. How might these variables affect foraging success? For example, flight in bright moonlight could be very risky for bats in the presence of owls. If it is very windy, flying and catching insects could be difficult. If it is raining, besides getting wet and cold, all of the falling raindrops could make it difficult to locate insects, if indeed the insects were still active in the rain. Low temperatures could result in both reduced insect activity, and in unacceptable loss of body heat during flight. These constraints, among others, result in hour-to-hour variation in activity at any given site, as well as night-to-night and seasonal variation [Citation3,Citation6]. Variation in activity among years is more rarely documented. The one study we are aware of monitored bat activity in the temperate climate of New Zealand in various seasons during 2 years and found that, although there were no significant changes in bat activity from 1 year to the next, there were major seasonal differences that were due primarily to changes in prey availability and nocturnal minimum temperatures [Citation5].
Long-term studies of neotropical bat activity from a variety of sites would clearly be useful in ecological evaluations and conservation efforts, but unfortunately are rarely reported. One such study, using acoustical recordings of insectivorous bats, was conducted within a few kilometres of our study sites, but over an elevational gradient on the Caribbean slope of Costa Rica [Citation14]. The most important variables they documented were elevation and temperature. In general, diversity and activity decreased with increasing elevation and decreasing temperature. However, surprisingly, diversity and activity were highest at middle elevations, rather than the lowest elevation as predicted.
We suspected that aerial insectivores in a tropical premontane forest, where cool temperatures and frequent rainfall prevail at night, should be especially sensitive to weather-related variables, and also to brightness of moonlight. We expected to encounter hour-to-hour, night-to-night, seasonal, and year-to-year changes in activity, correlating with changes in weather conditions and the phase of the moon. Because continuing effects of global and local climate change have impacted several important groups of vertebrates in Monteverde, including bats [Citation15], changes in population sizes and species composition might be anticipated during a period of this length.
We are not aware of any published studies that have examined how weather-related variables affect bat activity at tropical premontane forest sites. Long-term monitoring at such a site should provide valuable data. The closest approach to such a study is the one cited above [Citation14], which, however mainly considered elevation and temperature, but not other weather-related variables. A study in the United States produced similar results as regards temperature and elevation, and also found a negative correlation between bat activity and insect biomass [Citation16]. We suspect these variables and others may have similar effects in various life zones of the world, although much additional research will be required to establish these trends.
Herein, we explore the following general questions:
How many species of insectivorous bats can be detected acoustically at a single site in a tropical cloud forest?
Are bats equally diverse and active at different sites, some close, one several km distant, based on simultaneous recordings?
How do moonlight conditions, weather-related and seasonal variables, hour of night and presence or absence of other bat species affect the activity and diversity of bats recorded?
How do bat diversity and activity vary on time scales greater than hourly?
Did bat diversity and activity change over the ten-year period?
Material and methods
Our primary study site (PSS) was in Monteverde, Costa Rica, at 10.3092° N, 84.8135° W, 1,375 m.a.s.l. elevation. The study area is described in LaVal and Fitch [Citation17] and in LaVal [Citation15]. The physical environment of Monteverde as well as its biology was covered in considerable detail by Nadkarni and Wheelwright [Citation18]. Briefly, the study site is located in Tropical Premontane Wet Forest, but is within the Monteverde community; thus, it consists of forest patches separated by scattered residences, hotels, restaurants, and other businesses. The Bajo del Tigre sector of the Children’s Eternal Rainforest (Bosque Eterno de los Niños) extends to the southern edge of the study site. We note that this is the largest private reserve in Costa Rica, with an area of more than 20,000 Ha. The proximity of this reserve suggests we should expect that most of the local species would be present, especially since there are nearby open areas required by some insectivorous bats. The site itself consists of an overgrown fruit orchard with mixed citrus, bananas, guava, kas, loquat and avocado with a few native trees interspersed, and an adjacent abandoned, overgrown kitchen garden of herbs and vegetables. The orchard/garden is surrounded by forest in various stages of regeneration with a number of old-growth trees. This forest has never been clear-cut, but was subjected to selective timber harvest in the 1940’s. Size of the orchard/garden clearing is approximately 75 m by 100 m. It slopes steeply to a small permanent stream. The highest point is to the north, where a dense, tall forest serves as an effective windbreak for the trade winds that often blow strongly from December through March.
Between November 2000 and December 2006, we monitored all night on a total of 1,002 nights, during 61 months (some months were skipped during 2003–2006). In addition, continuous recording was carried out from 27 June 2008 to 30 August 2008, and again during July and August of 2009 and 2010, and finally during July 2013 and 2015 for a total of 195 additional nights. Although recording was carried out for 10 years, far fewer nights were recorded in the later years as compared to the first 3 years. All monitoring was done passively with a single Anabat II detector (Titley Electronics) connected to a computer. The detector was mounted in an open window of a house, and pointed at approximately a 60° angle from the horizontal at the adjacent orchard/garden complex surrounded by forest. Weather and other conditions permitting, the computer and Anabat were turned on at dusk and off at dawn. The Anabat detector site was about 200 m west of one of the main bat netting sites used by LaVal [Citation15]. Bat passes were recorded on a nightly data sheet, with a bat pass being a single pass by one individual through air space as sampled with a bat detector [Citation19]. A bat pass may consist of a few to dozens of individual calls, as the Anabat software automatically records the pass as a file once calls can no longer be detected.
It is clear that we must be careful in how we interpret the number of bat passes. Hayes [Citation20] discussed common assumptions in bat pass studies. The number of acoustically determined bat passes is certainly a measure of the amount of foraging activity at a site being monitored, but it gives little information about the actual number of bats present, although it does tell us the relative foraging time of different species within the area sampled by the detector, as opposed to those just passing by. If ten species are recorded during one night, then theoretically there may have been only ten individual bats present, although frequently the calls of two or three individuals of the same species, or calls of two or more species, are recorded simultaneously. We cannot expect the various species to be recorded with equal precision, since many variables act to prevent that, like distance from the microphone, flight speed, height above the ground, proximity to vegetation, and relative loudness of the bats’ calls [Citation20]. In addition, seasonal variation in humidity may affect the number of bat passes recorded. With respect to identification of bat species, atmospheric attenuation caused by variations in humidity does indeed occur, but Goerlitz [Citation21] demonstrated that this would be critical when identifications are made by computer algorithms, but less so when made visually by an experienced bat biologist.
Identification of bat calls was based on visual examination of calls using Analook software. Bat calls were identified using published Anabat call sequences, e.g. [Citation22], plus identified recordings supplied by B. Miller and M. O’Farrell to identify bat calls, as well as our own recordings of species not recorded by those authors. We could not reliably distinguish between the calls of Tadarida brasiliensis and Nyctinomops laticaudatus, so we combined the calls of these two species, although N. laticaudatus has never been captured in the Monteverde area, and only once in Costa Rica. Approximately 5% of calls were unidentifiable. In order to avoid inconsistent results due to variation in sensitivity among microphones, the same Anabat II unit and microphone were used for all recordings at the PSS until the original unit failed in December 2006 and was replaced. Before the unit failed, it had been tested alongside the backup unit to ensure both were equally sensitive. The sensitivity setting on both units was kept at “6”.
Although data collection was concentrated at the PSS, we had the opportunity during one period to make simultaneous recordings at the PSS and at a site in a very different life zone near Monteverde, so we could make direct comparisons. This second site was monitored simultaneously with the primary site during December and January 2003–2004, April and May 2004, and August 2004. This site, at a commercial tourist development called Selvatura, is located about 4 km northeast of the PSS at 10.2012° N, 84.4754° W, 1,620 m.a.s.l. elevation on the Caribbean slope. Forest type is Lower Montane Rainforest, with an estimated annual rainfall of at least 4,000 mm. The Selvatura site is adjacent to the 20,000 ha Children’s Eternal Forest, and is in relatively pristine condition, except for the developed area at the resort center. We also opportunistically made recordings on one or more nights at several other sites, just to see how results might change from site to site on these individual nights. At two sites we made recording for consecutive three-day periods simultaneously with recordings at the PSS. One, the Tobi House, is located about 400 m north and 100 m higher in elevation than the primary site. The second site, the Monteverde Butterfly Garden, is about the same distance west of the PSS, and at slightly lower elevation. Weather conditions can be considered the same at these three sites. Single night recording sessions at a nearby site (the Vargas House, close to the Monteverde Butterfly Garden) and at the San Gerardo field station in the Children’s Eternal Forest were also carried out simultaneously with recordings at the PSS. San Gerardo is at 1,100 m.a.s.l. in Premontane Rainforest, although overgrown pastures and secondary forest surround the station. Since much of the between year and between sites data was gathered opportunistically, and limited by instrument availability and observer schedules, the analyses of the data are limited in many cases to anecdotal summaries, and exploratory analysis, in the sense of Tukey [Citation23], rather than statistically rigorous hypothesis testing. Given the limited data on bat communities in the montane neotropics, we feel that the information, although incomplete, is valuable.
The bat pass data was recorded along with estimated wind and cloud conditions, rainfall timing, hour of moonset or moonrise, species identification, and time divided into hourly periods. The PSS site was shaded by surrounding forest when the moon was low in the sky, but otherwise fully open to moonlight. At the PSS, daily rainfall and temperature data were collected about ten meters from the Anabat detector. Average annual precipitation (for the calendar year) at this site from 1980 through 2010 was 2,893 mm (30 year average – our data). For this study, a year was recorded as the period from November of one year through October of the following year, which best fits the local seasons. These seasons are defined by local changes in weather conditions, and cannot be compared to temperate zone seasons.
For the purposes of logistic regression analysis, the data was categorized. Phase of the moon was first quarter, full, third quarter, or new. Wind was calm, variable, or windy. Rain was none, light, or moderate (which included the heaviest rains). Although it might have been desirable to have electronic, automatic recording of wind velocity and hourly rain amounts, such equipment was not available to us. The approximately 12 hours of the night were divided into three periods, the first 3½ hours, the second 5 hours, and the final 3½ hours. Although the original intent was to divide the night into three equal time periods, activity was so reduced during the middle of the night that we decided to make that period longer. Longer or shorter nights would simply include more or less time in the first or the last period. We considered the seasons to be “Windy-Misty” (1 November–15 January); “Dry” (16 January–10 May); and “Wet” (11 May–31 October) [Citation24]. The “Windy-Misty” season is a transition from the rainy season to the dry season, during which high winds with blowing light rain are frequent. During the “Dry” season it is less windy, with less, widely scattered and variable rainfall. During the “Wet “ season daily rainfall is the rule. The dates given are averages based on 30 years of data at the PSS. Although temperatures are an important variable in many studies, in Monteverde nighttime temperatures vary relatively little from night to night or month-to-month [Citation24]. Our 30 years of temperature data and bat netting convinced us that these almost non-variable temperatures had no effect on bat activity.
For the three most abundant bat species (Myotis nigricans, M. pilosatibialis, and Eptesicus brasiliensis) we used stepwise multiple logistic regression to model the likelihood of observing the species, as a function of a set of independent continuous and categorical variables, following methods outlined by Hosmer and Lemeshow [Citation25]. We chose the year 2001–2002 as a representative one-year data set for the logistic regression analyses because it generally contained the largest sample sizes (Supplemental Material). For purposes of analysis, we classified bat species as simply being present or absent at the observation point during a sampling period. The analyses were conducted in R 2.14.12 [Citation26]. The independent variables we examined are wind, rain, the phase of the moon, time of the night, season of the year, and the presence and number of passes of other bat species. Exponentiating a model coefficient gives the odds ratio of observing the outcome of interest for one value of the independent variable relative to the reference value. Specific elements of model construction and interpretation are discussed in the Results section.
Results
Alpha diversity
There are at least 26 species of aerial insectivorous bats in the study area [Citation15]. The approximately 250,000 identified bat passes represent the calls of at least 20 species, probably more, since calls of two species were combined. At least six additional species of aerial insectivores were captured by us in or near the study area but were not recorded acoustically at the PSS. Of the 20 species that we observed acoustically, nine were Vespertilionidae, six were Molossidae, three were Emballonuridae, and two were Mormoopidae. The vast majority of individuals were of three vespertilionid species: Myotis nigricans (110,479 observed passes; 56.79% of the total), M. pilosatibialis (56,320 passes; 28.95% of the total), and Eptesicus brasiliensis (19,230 passes, 9.89% of the total). The complete species list and the observed passes for 2001–2002, 2002–2003, and 2003–2004 are provided in Supplemental Materials. After the third year of sampling no additional species were added to the list.
Relative activity among sites
Although the same species occurred at both PSS and Selvatura, relative species activity differed greatly between the sites; however, the differences were not consistent during the three sampling periods – December–January, April–May, and August 2004 (). Total bat activity fluctuated widely among the three data collection periods. In December–January average bat passes rose as high as 100 per night. In April–May they were lower with highs for common species around 40 per night. In August activity increased sharply, with bat passes of 160 to 260 per night for common species. In general, the four most common species were common at both sites, but proportionately different. Myotis pilosatibialis, however, was virtually absent at the PSS during December–January. Molossids and other species were consistently uncommon at the sites during these months, except during August at Selvatura, where Eptesicus fuscus reached a high average of 259 per night.
Figure 1. Bat activity by species at Monteverde in the Cordillera de Tilarán, Costa Rica, at the Primary Sampling Site (dark bars) and at Selvatura (pale bars) during three periods (January and February, April-May, and August) during 2003–2004. From the left species are Myotis pilosatibialis, Myotis nigricans, Eptesicus brasiliensis, Lasiurus ega, Pteronotus davyi, and Pteronotus gymnonotus.
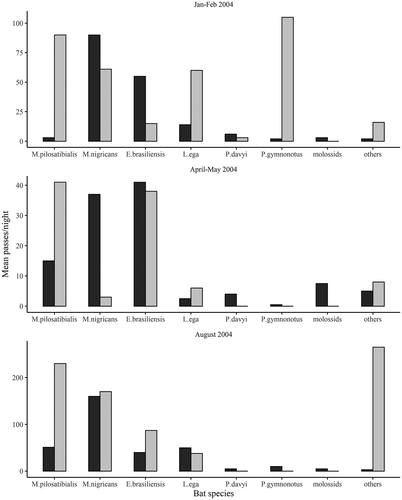
Myotis pilosatibialis, already uncommon at PSS by 2003, remained common at Selvatura in 2004 (). Eptesicus fuscus and Pteronotus gymnonotus were never common at PSS, but were seasonally abundant at Selvatura. P. gymnonotus and P. davyi were also much more commonly recorded at two other sites, the nearby Butterfly Garden and at the San Gerardo field station where on one night P. gymnonotus was the most common species recorded (380 passes). Since these last two sites were only monitored for one night, they are only mentioned to illustrate how drastically activity and species composition can differ among sites.
Effect of variables on activity
Univariate logistic regression analyses suggest that the likelihood of Myotis nigricans being present at the PSS is influenced by the phase of the moon, rain, wind, the time of night, the season of the year, the presence of Myotis pilosatibialis as a categorical variable, and the number of M. pilosatibialis passes as a continuous variable, so these were used in the forward stepwise construction of a multiple logistic regression model of the odds of M. nigricans being present (). At each step in model construction, the coefficients of independent variables already in the model do not change substantially, indicating that confounding due to collinearity among independent variables is not a problem [Citation25]. Although the odds ratio for {MOON_1} is 0.455, the confidence interval ranges from 0.352 to 0.589, so it’s best to say that M. nigricans is about half as likely to be present when the moon is in the first quarter than on nights around the new moon, the reference state. From the other odds ratios for phases of the moon, it appears that M. nigricans is about a third as likely to be present around the full moon, and about 2/3 as likely to be present when the moon is in the third quarter, in comparison to the darker nights around the new moon. Similarly, M. nigricans is several times more likely to be present when there is no rain, or light rain, than when the rain is stronger. Myotis nigricans is about ¼ as likely to be present at PSS when it is calm than when it is windy. In variable winds, this species is as likely to be present as when it is windy. Myotis nigricans is several times more likely to be present in the early evening than in the pre-dawn hours, while in the middle of the night they are as likely to be present as during the pre-dawn. They are several times more likely to be present in the windy/misty season than in the rainy season, but are as likely to be present in the dry season as the rainy season. Finally, M. nigricans is more likely to be present if M. pilosatibialis is also present. An increase of one count of M. pilosatibialis per hour increases the likelihood of M. nigricans being present by slightly less than 1%, but that means that thirty passes of M. pilosatibialis, would increase the likelihood of observing M. nigricans by about 25%.
Table 1. Stepwise construction of the multiple logistic regression model of factors influencing the likelihood of Myotis nigricans presence at the Monteverde study site. For the first and second model n = 2836, for the subsequent models n = 2685. Standard errors of the coefficients and odds ratios are in parentheses. MOON_1 is when the moon is in the first quarter, MOON_2 is around the full moon, MOON_3 is in the third quarter, all relative to nights around the new moon. RAIN_1 and RAIN_2 are no rain, and light rain, respectively, relative to moderate rain. WIND_1 and WIND_2 are calm and variable winds, respectively, relative to windy conditions. N_PART_1 are the first four hours of the evening, N_PART_2 are the hours between 10pm and 2am, both relative to the hours between 2am and Dawn. SEASON_1 is the windy-misty season (1 Nov −15 Jan), SEASON_2 is the dry season (16 Jan-10 May), both relative to the rainy season (11 May-31 Oct). MYOPIL is for a increase of one pass of Mysotis pilosatibialis. See the discussion of analyses in Results for details of interpretation
The multiple logistic regression model for the likelihood of observing M. nigricans at the study site () allows us to make the general statements about combinations of conditions. For example, M. nigricans are about 1/5 as likely to be observed at the study site when it is calm than when it is windy, and about ¼ as likely to be observed when the moon is full than in the darker nights around the new moon. Since these effects are independent, M. nigricans is only about 1/20 as likely to be observed on calm nights with full moon than on windy nights with new moon. Although the confidence interval about this estimate is broad (about 1/45 to 1/13), the effect is certainly clear. Similar calculations can be made for any combination of conditions.
The likelihood of Myotis pilosatibialis being present at the PSS is influenced by the same factors that influence the likelihood of observing M. nigricans at the site, but the importance of the factors differs between the species (). The phase of the moon has the largest impact on the likelihood of observing M. pilosatibialis, followed successively by time of night, wind, season, and rain. Finally, after these, if Myotis nigricans is present at the site, the chance of observing M. pilosatibialis is around ¾ that if M. nigricans is absent. There is no evidence of collinearity among the independent variables; the regression coefficients of independent variables already in the model remained stable with the addition of further variables. There were no significant interactions among independent variables. Myotis pilosatibialis is about 1/3 as likely to be observed around the full moon as in the dark of the moon, is several times more likely to be observed in the evening or the middle of the night than in the hours before dawn, is about half as likely to be observed in calm weather than when winds are variable or strong, is about half as likely to be observed in the windy-misty season (and 2/3 as likely in the dry season) as in the rainy season, and 1.5–2 times as likely to be observed on nights when it is not raining or raining lightly, as on nights with moderate rain. As described above for M. nigricans, the regression coefficients and odds ratios in can be readily combined to calculate the relative likelihoods of observing M. pilosatibialis under various combinations of conditions.
Table 2. Stepwise construction of the multiple logistic regression model of factors influencing the likelihood of Myotis pilosatibialis presence at the Monteverde study site. For the first and second models n = 2696, for the subsequent models n = 2685. Standard errors of the coefficients and odds ratios are in parentheses. Independent variables as described in . MYONIG is Myotis nigricans present relative to M. nigricans absent
The likelihood of observing Eptesicus brasiliensis is influenced by several of the environmental factors examined, but the importance of the factors differs from either of the Myotis species (). Rain, however, had no effect. Briefly, E. brasiliensis is several times more likely to be observed in the evening than in the middle of the night or in the pre-dawn hours, is about half as likely to be observed around the full moon as at other phases of the lunar cycle, is several times more likely to be observed in the windy-misty season and the dry season than in the rainy season, and is about half as likely to be observed in calm conditions as when conditions are windy, even if the winds are variable. Interestingly, E. brasiliensis is about half as likely to be seen when M. nigricans is observed at the site as when it is absent. Again, the regression coefficients of each variable in the model remained stable as subsequent variables were added, so confounding collinearity is not an issue, and no significant interactions among the variables were observed, so calculation of the likelihood of observing E. brasiliensis for various combinations of conditions can be pursued as outlined above for M. nigricans.
Table 3. Stepwise construction of the multiple logistic regression model of factors influencing the likelihood of Eptesicus brasiliensis presence at the Monteverde study site. For the first model n = 2847, for the second and third n = 2696, and for subsequent models n = 2685. Standard errors of the coefficients and odds ratios are in parentheses. Independent variables as described in . MYONIG is Myotis nigricans present relative to M. nigricans absent
In addition to influencing the activity of the three most common species, wind, rain, and moonlight appear to affect overall activity in the insectivorous bat community at the primary study site. Wind had a positive effect on the total number of bat passes ()). During a two-year period, bat passes almost always were greater on windy nights (P < 0.001; Wilcoxon Signed Rank test). Rain had a negative effect on the number of bat passes (). During the rainy season months of 2001–2002, bat activity was depressed on all these nights, even after the rain had ceased. Bat activity was significantly less, compared to average bat passes in those months ()), both during rain and for overall activity on these rainy nights (Wilcoxon Rank Sum tests, P < 0.001). Bright moonlight had a negative effect on the number of bat passes. A sample lunar month is shown in ). Although it is clear that bat activity is greatly reduced on nights with bright moon, we saw considerable day-to-day variation in the number of passes that probably reflects the negative effects of nocturnal rainfall and overcast conditions on some of these nights.
Figure 2. Effects of wind, phase of moon, and time of night on bat activity at the Primary Sampling Site at Monteverde in the Cordillera de Tilarán, Costa Rica. All species combined. A. Bat activity on paired windy (open circles) and calm (filled circles) nights during the 2001–2002 year. B. Bat activity during the April-May, 2002 lunar cycle. C. Mean hourly bat passes (± one st. dev.), for all nights combined during the 2001–2002 year
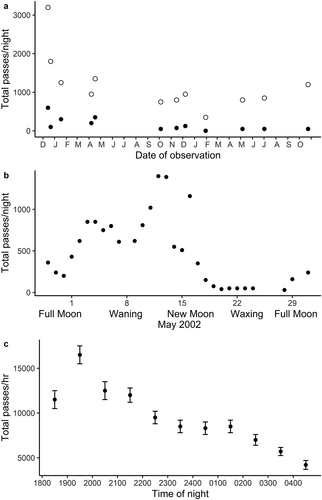
Figure 3. Differences in bat activity among years at the Primary Sampling Site at Monteverde in the Cordillera de Tilarán, Costa Rica. A. Monthly mean bat passes per night, all species combined, for three years (2000–2001, 2001–2002, and 2002–2003). B. Decline in bat activity, all species combined, for the rainy season months of July (filled circles) and August (open circles) from 2001 to 2010
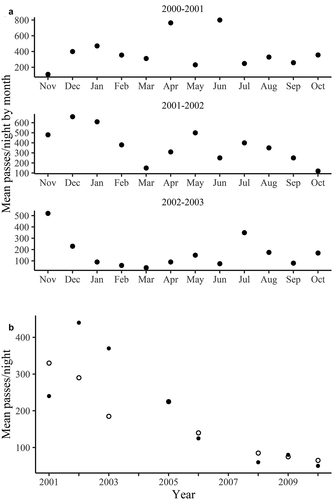
Table 4. Bat activity on rainy (but not misty) nights in 2001 and 2002 at the Primary Sampling Site at 1350m in Monteverde in the Cordillera de Tilaran, Costa Rica
Effect of various time scales on activity
Bat activity varied greatly on all time scales for which data were recorded. On an hourly basis, activity peaked in the second hour after dusk, and then gradually decreased until dawn ()). The minimum number of passes, which on the average was between 0300 and 0400, was one-third the number at peak activity between 1900 and 2000. Bear in mind that these are total figures for 1 year, and that on some nights the pattern was quite different. However, this does represent the general trend observed on most nights.
On a monthly basis bat activity showed significant changes from month to month and year to year. These are exemplified by the 3 years shown in ). In none of the months of these years were activity levels similar for all 3 years. In November 2002 bat activity was greater than in November of the prior and following year but in all other months activity was much lower during 2002–2003. During these 3 years, at least, there are no marked trends evident in monthly bat activity, although March and September seemed to be the least active months each year. The months with greatest bat activity differed among years. During the following 3 years (2004–2007), average activity dropped to less than half that of the previous three, although recall that some months were not represented in the data for those years, having an unknown affect on activity patterns.
On an individual species basis, nightly activity patterns look quite different among the species. We examined the four most common species recorded in 2001–2002 ()). Myotis nigricans and M. pilosatibialis seem to have complimentary activity patterns. Myotis nigricans usually begins the night at a high activity level, becomes increasing active, then drops before rising somewhat to maintain a moderately high activity level until dawn. Myotis pilosatibialis begins at a low activity level, which increases steadily until the middle of the night, then declines gradually. Eptesicus brasiliensis begins at a high level very early, drops off very sharply, and stays at a low level the rest of the night. These patterns are reflected in the logistic regression models discussed earlier. Lasiurus ega, by contrast, begins at a very low activity level and gradually increases until midnight, decreases somewhat, then increases again before dawn.
Figure 4. Effects of time of night, season, and year on the activity of the most common insectivorous bats species at the Primary Sampling Site at Monteverde in the Cordillera de Tilarán, Costa Rica. A. Bat activity by time of night for four common species of bats (Myotis nigricans, M. pilosatibialis, Eptesicus brasiliensis, and Lasiurus ega) during 2001–2002, as indicated by number of passes per hour. Note differences in y-axis scales. B. Bat activity (mean passes/night) by month during five years for the five most commonly detected bat species (Myotis nigricans, M. pilosatibialis, Eptesicus brasiliensis, Lasiurus ega, and Pteronotus davyi)
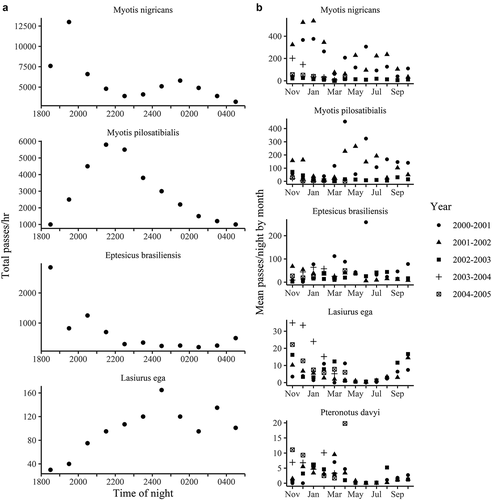
Monthly activity patterns of individual species at the PSS over the course of the first three or 5 years were extremely variable. Five species produced enough data to be graphed ()). A close examination of the figures shows that these species were seasonally common, seasonally rare, or even seasonally absent.
During the 10-year period, the more common species varied dramatically in their relative percentage of the total bat passes. During the first 3 years, two species of vespertilionid bats (Myotis pilosatibialis and M. nigricans) accounted for 85% of the passes, and another vespertilionid (Eptesicus brasiliensis) accounted for another 10%. The three species only accounted for about 70% of the passes in 2005 and 2006, and by 2008 and 2009, they had dropped to about 60%, with a fourth vespertilionid (Lasiurus ega) and a molossid (Molossus alvarezi) accounting for much of the remaining activity. Notably Myotis pilosatibialis, which accounted for 46% of the passes in 2001, declined each year thereafter until it was <0.2% in 2010. Although many species showed year-to-year fluctuations in activity, including some obvious trends towards reducing or increasing their percentage, only M. pilosatibialis demonstrated such a drastic decline over the 10-year period. Eptesicus brasiliensis gradually increased its percentage from 10% early in the study to 40% in 2009, whereas M. nigricans decreased gradually from 57% to only 13% in 2009. Meanwhile, Lasiurus ega increased its percentage from only 1.5% to nearly 16% in 2009. Molossids also increased, beginning at about 2% and reaching 12% in 2009. Recall that after 2003, far fewer nights were recorded, but for these species normally common the year round, these trends should still be accurate. Emballonurids are uncommon at this elevation, although Diclidurus albus was consistently present throughout the study, with a maximum of 148 passes recorded in 2001–02. The mormoopids Pteronotus gymnonotus and P. dayvi were also consistently present in low numbers.
Activity decrease over ten years
An almost constant decrease in the average number of bat passes per night in the months of July and August from 2000 to 2010 is shown in ). July and August were the only months for which we had data for the later years, and we lack data for those months for 2004 and 2007. July nightly average passes dropped from a high of nearly 450 in 2002 to a low of about 50 in 2010, and the change in August passes was only slightly less dramatic. Meanwhile, alpha diversity remained virtually unchanged with no apparent changes in the quality or size of the available foraging habitat at PSS during this period. Data from July 2013 revealed an average of 71 nightly passes, marginally above that of 2010, but was up to 209 in 2015.
Discussion
Alpha diversity
The number of species detected acoustically at Monteverde (20) may be characteristic of low and middle elevation sites in the tropics. For example, Jung and Kalko [Citation8] recorded 25 species in lowland Panama; MacSwiney et al.[Citation27] recorded 26 in Yucatan, México, and Appel et al. [Citation28] mentioned 19 near Manaus, Brazil, but they did not report how many species they actually recorded acoustically. In the Old World, Milne et al. [Citation3] recorded 24 species in tropical Australia. Unfortunately, little has been published based on long-term acoustical recording in the tropics. Nevertheless, data from Mexico, Costa Rica, Panama, Brazil, and tropical Australia suggest that insectivorous bat species richness among co-existing species at a location in the seasonally wet tropics does not fluctuate widely on a geographical basis (although Paleotropical sites may have greater overall insectivorous species diversity) nor between low and medium elevations. Clearly more research with standardized sampling methodology is needed.
The fact that a few species accounted for most of the passes at all sites sampled suggests that these species have adaptations and/or behaviors that result in their choice of these specific sites for long-term foraging. These species may also be much more common than the less-frequently recorded species, but our data neither confirm nor deny that possibility. High-flying bats like molossids and Diclidurus albus may be underrepresented in our bat pass tally simply because they have larger or higher foraging territories and thus spend less time within range of the microphone. Typically, with these species, multiple sequences of recordings that suggest a bat is foraging near the microphone are rare.
Relative activity among sites
Since the variables tested here vary little among the nearby sites of PSS, Tobi House, Butterfly Garden, and Vargas House, it is not surprising that overall diversity and activity level is not greatly different among them. However, relative species abundance is sometimes quite different. Clearly there are other variables not measured here that affect relative species abundance, including structural complexity of each site and how that relates to the foraging behavior and other adaptations of each species. In the case of San Gerardo and Selvatura, as compared to PSS, there are major differences in wind and rain among the sites, due to change in altitude and location. In addition, temperature is higher than PSS at San Gerardo at about 1200 m and lower at Selvatura at about 1700 m. Thus, predictably, there are greater differences in diversity and relative species abundance. In Switzerland at 5 sites studied, differences in landscape structures were found to be a viable explanation for differences in relative abundance among the eight species encountered [Citation29]. Perhaps this helps explain the differences among nearby sites in Monteverde. Another potential factor is local and elevational movements by bats tracking seasonal changes in food availability. Seasonal changes in the phenology of fruiting and flowering plants on which bats forage in Monteverde have been documented [Citation30]. We have been netting bats in Monteverde during most years since 1973 and have frequently observed changes in species composition and diversity among netting sites in the local area (unpublished data).
Effect of variables on activity
Wind has been shown to have a negative effect on bat activity by Russo and Jones [Citation31], but has received relatively little attention. Verboom [Citation12] demonstrated that Pipistrellus pipistrellus activity was concentrated on the leeward side close to treelines (which we interpret as windbreaks) under windy conditions, and was concentrated closer to the trees at higher wind speeds or higher incidence angles. He also showed that insect activity was higher on the leeward side of the windbreak, a pattern documented previously by Lewis [Citation32]. Although we have no data on insect abundance, we note that wind speeds in the open in Monteverde often exceed 50 km/h, whereas at PSS there is no sustained wind due to the windbreak effect of the nearby forest. We assume that insect abundance is therefore higher at PSS than in windy sites, and that, furthermore, flight conditions for bats are much more favorable at PSS, resulting in higher bat activity on windy nights, as data show for that site ()).
Most studies in which the effect of rain was documented show that rain has a negative effect on bat activity, e.g. [Citation4,Citation13,Citation33]. The consensus is that bats will forage in light rain, but cease flight as the rain gets heavier. In our study we found that moderate and heavy rain resulted in a great reduction in bat activity of the most common species (, ), and on some rainy nights there were zero passes of insectivorous bats. We had expected that on nights with heavy rain before midnight bat activity would increase to normal levels after the rain had ceased. This was not the case. Perhaps the heavy rains earlier in the night had a more persistent reducing effect on insect activity, due to wet vegetation, which in the forest drips steadily for several hours after the rain ceases. In a recent study Voigt et al. [Citation34] demonstrated that Carollia sowelli, a frugivorous species found in Monteverde, exhibited a doubled metabolic rate in flight when wet or exposed to rain. Clearly, bats can be expected to avoid or at least minimize flight under these conditions.
Many investigators have tried to demonstrate a relationship between brightness of the moon and bat activity, with results ranging from no effect to a very strong effect (e.g. [Citation35]). The three most commonly observed species in our study were substantially less active in the brightly lit nights around the full moon than in the dark nights around the new moon (, , ). Although studies of several Neotropical species have shown negative reactions to moonlight (for example, the tropical gleaner, Lophostoma sylvicolum [Citation10], none of these were aerial insectivores. However, Holland et al. [Citation36] were not able to demonstrate any effect of moon phase on the insectivorous Molossus molossus. Recently, Appel et al. [Citation28] tested five species of insectivorous bats (one vespertilionid, 4 emballonurids) for the effect of moonlight using ultrasonic detectors in Brazil. Of these, only Myotis riparius, a vespertilionid, occurs in our study area, and it was the only species with a clear-cut negative reaction to moonlight intensity. Recall that most of our data are from vespertilionid bat species. A clear-cut example of moonlight having a negative effect on a wide spectrum of tropical insectivorous bats (and on their prey insects) was in Ivory Coast, West Africa [Citation7]. In Chile, Vásquez et al. [Citation37] found that the four vespertilionid bats they studied all reduced their activity on bright moonlet nights, but a molossid did not. In their review, Saldaña-Vásquez and Munguía-Rosas [Citation38] suggest that lunar phobia is more pronounced in the tropics and in bat species whose foraging space is more exposed to moonlight. They also note an overall negative response to moonlight. As noted long ago [Citation39], insect activity as sampled with a suction trap follows the lunar cycle, being highest in the dark nights around the new moon. We have for many years made the observation that far more insects are attracted to lights in Monteverde during the dark period of the moon, although we have no quantitative data. Also, there are potential avian bat predators (various species of owls) in Monteverde that could profit from bright moonlight. Although we have not observed predation on bats here, it has been well documented elsewhere in Costa Rica [Citation40], (LaVal, unpubl. obs., J. Wolfe, unpubl. obs.). Scarcity of insect prey as well as the risk of being captured by visually oriented predators may be sufficient to discourage bats from foraging in the open during periods of bright moonlight. Unfortunately, we have no data to suggest just where they do forage under these conditions, although Reith [Citation41], using multiple bat detectors, demonstrated that Myotis yumanensis in New Mexico, USA, foraged in shady sites during bright moon, but moved to more open areas when the moon was dark. If bats in Monteverde chose that option, there are plenty of forested areas nearby where they could forage in the shade.
Our analyses of the activity of the three most commonly observed species show clearly (,,) that increased activity of Myotis pilosatibialis increases the likelihood of observing Myotis nigricans, but the presence of M. nigricans reduces the likelihoods of observing M. pilosatibialis and Eptesicus brasiliensis. Our data do not explain this phenomenon, but casual observations suggest some possibilities. We believe M. pilosatibialis forages within a few meters of the ground (frequently captured in nets) whereas M. nigricans forages in a wider vertical space (much less frequently captured even where common). The presence of M. nigricans may “discourage” M. pilosatibialis, which in turn moves to another foraging site. Such interactions may be ameliorated by the different times of night in which they are more likely to be observed acoustically. Similar reasoning might apply to E. brasiliensis, which usually flies higher in the canopy (rarely captured in nets even where common) than M. nigricans. A detailed study in French Guiana demonstrated how space, food, and/or time partitioning allowed similar species of frugivorous and nectarivorous bats to coexist at the same site [Citation42].
Effect of various time scales on activity
Diversity and activity were highly variable on all time scales we examined. Although the variables we studied must account for much of this variation, many other factors may also be at work, including the bats’ perceived hunger and metabolic needs, seasonal changes of all kinds, insect life cycles, interactions with predators and other bat species, and reproductive needs. Presley et al. [Citation43], reporting on phyllostomid bat activity at a site in Amazonian Peru, noted that season had little effect on activity patterns, but that habitat differences did result in changes of activity patterns for some species. Estrada-Villegas et al. [Citation44] showed that “ … climate at large scales explained most of the variation in individual species abundance”, based on acoustic monitoring in Panama.
Activity decrease over ten years
Based on the reduction in bat activity, we suggest that the bat population declined during our ten-year study period, but our data was not designed to examine that hypothesis. Recall that the data represented by Figure 8 was collected during nightly recording during July and August during the ten year period, simply because those were the sampled months in the later years. However, our mist-netting efforts between 2005 and 2020 in Monteverde suggest that M. pilosatibialis, the only insectivore commonly captured in mist nets here, was much less common locally than during previous years of netting. Year-round monthly netting in 1999 and 2020 [Citation15] and additional unpublished data of our own support an overall reduction in populations of the common species of insectivorous bats. One possible explanation for this decline in activity is the great expansion of the tourist industry, and consequent local population growth, in this period, which has resulted in the installation of hundreds of bright lights. As documented by Rich and Longcore [Citation45], streetlights may decimate insect populations. A review by Patriarca and Debernardi [Citation46] gave many reasons why this might be the case. If true in Monteverde, insectivorous bat populations would be negatively impacted, even though there are no streetlights within 100 m of PSS (but several within 200 m). Another possible explanation is that perhaps most of the bats have abandoned this foraging site in favor of foraging near streetlights. A recent study carried out in Monteverde showed more than twice the bat activity at streetlights than in similar unlighted sites [Citation47]. Finally, we note that declines of insect populations worldwide, documented by Sánchez-Bayo and Wyckhuys [Citation48] appear to be also occurring in Monteverde, based on our personal observations over 40 years of residence here. Elsewhere in lowland Costa Rica, this phenomenon has been amply documented and discussed by Janzen and Hallwachs [Citation49]. Clearly a decline in insect abundance might be a contributing factor to local declines in insectivorous bat activity. The decline in bat activity appears to have slowed during the last 3 years shown, and hopefully will at least stabilize, which is suggested by the slightly increased bat activity during July 2013 and 2015. Investigations at broad geographic scales of both insect abundance and bat communities will obviously be of ecological and conservation interest.
Conclusions
The vast and unpredictable variation in relative activity on a temporal scale and among species suggests that insectivorous bats, rather than being creatures of habit, react continuously to local geographic, temporal and seasonal variation in insect populations and select optimal foraging sites as long as other factors do not intervene. The fact that many species are absent or mostly absent from our primary study site during portions of the year suggests local movements that, on a steep mountain slopes like those around Monteverde, probably consist of altitudinal migrations, presumably on an annual basis.
Author contributions
R.K. LaVal conceived and designed the study, carried out the sampling and much of the data analysis, and wrote most of the manuscript. R.O. Lawton performed the logistic regression analyses, wrote portions of the manuscript, and provided essential editorial advice and aid in revising the manuscript. As a long-time researcher in Monteverde he is familiar with many of the concepts discussed in the paper.
Supplemental Material
Download MS Word (14.6 KB)Acknowledgments
We thank Bruce Miller and the Wildlife Conservation Society for loan of Anabat equipment used through 2006. Michael O’Farrell and Bruce Miller helped identify bat calls. Michael Lawton aided in the multiple logistic regression analysis. Robert Timm helped in various ways with early drafts of the manuscript. Eight unnamed reviewers provided editorial assistance. Ragde Sánchez assisted with the translation of the resumen. The entire LaVal family exercised stentorian patience as rain and cold wind blew in the open window night after night as bat calls were being recorded. Our thanks to Anabat and Panasonic for designing equipment that would continue to function under these conditions. This research was aided by a grant from the Centro Cientifico Tropical (Tropical Science Center) in San Jose, Costa Rica. There are no relevant financial or non-financial competing interests to report. This paper is dedicated to the late Margaret LaVal who supplied much needed moral and logistic support throughout this project and our research at Monteverde for more than four decades.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Frick WF. Acoustic monitoring of bats, considerations of options for long-term monitoring. Therya. 2013;4:69–78.
- Ford WM, Britzke ER, Dobony CA, et al. Patterns of acoustical activity of bats prior to and following white-nose syndrome occurrence. J Fish WL Mgt. 2011;2:125–134.
- Milne DJ, Fisher A, Rainey I, et al. Temporal patterns of bats in the top end of the Northern Territory, Australia. J Mammal. 2006;86:909–920.
- Thies W, Kalko EKV, Schnitzler H-U. Influence of environment and resource availability on activity patterns of Carollia castanea (Phyllostomidae) in Panama. J Mammal. 2006;87:331–338.
- O’Donnell CFJ. Influence of season, habitat, temperature, and invertebrate availability on nocturnal activity of the New Zealand long-tailed bat (Chalinolobus tuberculatus). NZ J Zool. 2000;27:207–221.
- Scanlon AT, Petit S. Effects of site, time, weather and light on urban bat activity and richness: considerations for survey effort. CSIRO Wildl Res. 2008;35:821–834.
- Meyer CF, Schwarz JCJ, Fahr J. Activity patterns and habitat preferences of insectivorous bats in a West African forest-savanna mosaic. J Trop Ecol. 2004;20:397–407.
- Jung K, Kalko EKV. Where forest meets urbanization: foraging plasticity of aerial insectivorous bats in an anthropogenically altered environment. J Mammal. 2010;91:144–153.
- Hecker KR, Brigham RM. Does moonlight change vertical stratification of activity by forest-dwelling insectivorous bats? J Mammal. 1999;80:1196–1201.
- Lang AB, Kalko EKV, Römer H, et al. Activity levels of bats and katydids in relation to the lunar cycle. Oecologia. 2006;146:659–666.
- Duran AA. Influence of abiotic factors on population of Carollia perspicillata (Lineaus 1758) (Mammalia: Chiroptera). Acta Zool Lilloana. 2018;62:24–30.
- Verboom B. The use of edge habitats by commuting and foraging bats. IBN Sci Contri. 1998;10 1–123 . DLO Inst Fores and Nat Res. Wageningen, Netherlands.
- Erickson JL, West SD. The influence of regional climate and nightly weather conditions on activity patterns of insectivorous bats. Acta Chiropt. 2002;4:17–24.
- Arias-Aguilar A, Chacón-Madrigal E, LaVal R, et al. Diversity and activity patterns of aerial insectivorous bats along an altitudinal gradient in a tropical forest in Costa Rica. Hystrix 2020. Available from: http://www.italian-journal-of-mammalogy.it
- LaVal RK. Impact of global warming and locally changing climate on tropical cloud forest bats. J Mammal. 2004;85:237–244.
- Wolbert SJ, Zellner AS, Widden HP. Bat activity, insect biomass, and temperature along an elevational gradient. NE Naturalist. 2014;21:72–85.
- LaVal RK, Fitch HS. Structure, movements and reproduction in three Costa Rican bat communities. Occas Pap Mus Nat Hist Univ Kansas. 1977;69:1–28.
- Nadkarni NM, Wheelwright NT, editors. Monteverde: ecology and conservation of a tropical cloud forest. New York (NY): Oxford University Press; 2000.
- Kunz TH, Gustapson AW. Terms commonly used and misused in the literature pertaining to bats. Bat Res News. 1983;24:19–22.
- Hayes JP. Assumptions and practical considerations in the design and interpretation of echolocation-monitoring studies. Acta Chiropt. 2000;2:225–236.
- Goerlitz HR. Weather conditions determine attenuation and speed of sound: environmental limitations for monitoring and analyzing bat echolocation. Ecol Evol. 2018;8:5090–5100.
- Ochoa G, O’Farrell JM, Miller BW. Contribution of acoustic methods to the study of insectivorous bat diversity in protected areas from northern Venezuela. Acta Chiropt. 2000;2:171–184.
- Tukey JW. Exploratory data analysis. Reading (MA): Addison-Wesley; 1977.
- Clark KL, Lawton RO, Butler PR. The physical environment. In: Nadkarni NM, Wheelwright NT, editors. Monteverde: ecology and conservation of a tropical cloud forest. New York (NY): Oxford University Press; 2000. p. 15–38.
- Hosmer DW, Lemeshow S. Applied logistic regression. New York (NY): Wiley; 1989.
- R Development Core Team. R: a language and environment for statistical computing. Vienna; 2012. Available at: http://www.R-project.org.
- MacSwiney MC, Clarke FM, Racey PA. What you see is not what you get: the role of ultrasonic detectors in increasing inventory completeness in Neotropical bat assemblages. J Appl Ecol. 2008;45:1364–1371.
- Appel G, López-Baucells A, Magnusson WE, et al. Aerial insectivorous bat activity in relation to moonlight intensity. Mammal Biol. 2017;85:37–46.
- Kusch J, Schotte F. Effects of fine-scale foraging habitat selection on bat community structure and diversity in a temperate low mountain range forest. Folia Zool. 2007;56:263–276.
- Dinerstein E. Reproductive ecology of fruit bats and the seasonality of fruit production in a Costa Rican cloud forest. Biotropica. 1986;18:307–318.
- Russo D, Jones G. Use of foraging habitats by bats in a Mediterranean area determined by acoustic surveys: conservation implications. Ecography. 2003;26:197–209.
- Lewis T. Patterns of insects near a windbreak of tall trees. Ann Appl Biol. 1970;65:213–220.
- Weinbeer M, Meyer CFJ, Kalko EKV. Activity pattern of the trawling phyllostomid bat, Macrophyllum macrophyllum in Panama. Biotropica. 2006;38:69–76.
- Voigt CC, Schneeberger K, Voigt-Heucke SL, et al. Rain increases the energy cost of bat flight. Biol Lett. 2011;7:793–795.
- Ciechanowski M, Zajac T, Bitas AR, et al. Spatiotemporal variation in activity of bat species differing in hunting tactics: effects of weather, moonlight, food abundance, and structural clutter. Can J Zool. 2007;85:1249–1263.
- Holland RA, Meyer CFJ, Kalko EDV, et al. Emergence time and foraging activity in Pallas’ mastiff bat, Molossus molossus (Chiroptera: Molossidae) in relation to sunset/sunrise and phase of the moon. Acta Chiropt. 2011;13:399–404.
- Vásquez DA, Grez AA, Rodríguez-sanpedro A. Species-specific effects of moonlight on insectivorous bat activity in central Chile. J Mamm. 2020;101:1356–1363.
- Saldaña-Vásquez RA, Munguía-Rosas MA. Lunar phobia in bats and its ecological correlates: a meta-analysis. Mammal Biol. 2013;78:216–219.
- Williams CB, Singh BP. Effect of moonlight on insect activity. Nature. 1951;167:153.
- Stiles FG, Skutch AF. A guide to the birds of Costa Rica. Ithaca (NY): Cornell University Press; 1989.
- Reith CC. Insectivorous bats fly in shadows to avoid moonlight. J Mammal. 1982;63:685–688.
- Delaval M, Henry M, Charles-Dominique P. Interspecific competition and niche partitioning: example of a Neotropical rainforest bat community. Rev d’Ecologie (Terre et Vie). 2005;60:149–166.
- Presley SJ, Willig MR, Castro-Arellano I, et al. Effects of habitat conversion on temporal activity patterns of phyllostomid bats in lowland Amazonian rainforest. J Mammal. 2009;90:210–221.
- Estrada-Villegas S, McGill BJ, Kalko EKV. Climate, habitat and species interactions at different scales determine the structure of a Neotropical bat community. Ecology. 2012;93:1183–1193.
- Rich C, Longcore T, editors. Ecological consequences of artificial night lighting. Washington (DC): Island Press; 2006.
- Patriarca E, Debernardi P. Bats and light pollution. Centro Regionale Chirotteri, Turin. 2010 [cited 2018 Oct 7]. Available from: http://www.centroregionalechirotteri.org/download/eurobats/Bats%20and%20light%20pollution.pdf
- Frank TM, Gabbert WC, Chaves-Campos J, et al. Impact of artificial lights on foraging of insectivorous bats in a Costa Rican cloud forest. J Trop Ecol. 2018;35:8–17.
- Sánchez-Bayo F, Wyckhuys KAG. Worldwide decline of the entomofauna: a review of its drivers. Biol Cons. 2019;232:8–27.
- Janzen DH, Hallwachs W. Where might be many tropical insects? Biol Cons. 2019;233:102–108.
