ABSTRACT
Dwarf caimans (Alligatoridae: Paleosuchus palpebrosus and Paleosuchus trigonatus), are small crocodilians exhibiting cryptic behaviour and complex habitat use that occur throughout the Amazon region. Our goals were to evaluate individual home range, habitat occupancy and movement patterns where they occur in sympatry in relation to seasonal water-level variation. We tracked five P. palpebrosus and seven P. trigonatus using VHS radio transmitters along the shores of two streams directly influenced by the flooding pulse of the Purus River. Paleosuchus palpebrosus individuals moved greater distances on a daily basis and had larger home ranges than P. trigonatus, and the species had different responses to increases in water levels. Paleosuchus palpebrosus moved into flooded forests, as do their principle prey species. Conversely, larger P. trigonatus individuals usually remained near the main stream, and were relatively sedentary. Intraspecific home-range overlap was higher than interspecific overlap in both species. Thus, habitat occupancy patterns as a function of water-level variation might facilitate coexistence of the two species of dwarf caimans in the same location. This study shows that when living in sympatry under a seasonal flooding regime, Paleosuchus species show a degree of habitat partitioning evidenced by different daily movement rates, home-range sizes and home-range locations.
Introduction
Ecological theory suggests that two species using the same limited resource in the same manner cannot coexist in the long term [Citation1–3], and habitat is often considered an important factor limiting coexistence. Crocodilians have cryptic amphibious habits, remaining most of the time submerged or hidden in vegetation or burrows along the shoreline of water bodies [Citation4,Citation5]. Within the same general aquatic environments, different crocodilian species may occupy distinct microhabitats and this may facilitate their coexistence [Citation6]. Four crocodilian species (Alligatoridae: Melanosuchus niger, Caiman crocodilus, Paleosuchus palpebrosus and Paleosuchus trigonatus) occur in sympatry in several locations in the Amazon basin [Citation7–12]. Their coexistence has been suggested to be a result of food-resource partitioning [Citation13–15], differences in feeding behaviours [Citation16], nest-site choice [Citation17,Citation18] and different parental-care strategies [Citation19]. General patterns of habitat use have been documented for some Amazonian caiman species, especially the more conspicuous M. niger and C. crocodilus [Citation7,Citation8,Citation20] and to a lesser degree Paleosuchus species [Citation21]. However, it is not well understood to what extent Amazonian crocodilians living in syntopy partition the habitat they occupy, especially as a function of seasonal hydrological changes.
The two South American dwarf caiman species (P. palpebrosus and P. trigonatus) occur in syntopy along the shores of floodplain streams in the Amazon basin, whose extent and depth are largely controlled by the seasonal fluctuations of large-river water levels [Citation22–24]. Water-level variation affects crocodilian population densities, behaviour and diet [Citation5,Citation11,Citation25,Citation26]. Floodplain streams are subjected to predictable and long-lasting floods [Citation23,Citation27]. This causes a considerable increase in the availability of aquatic environments within flooded forests during the high-water period. However, it is not known to what extent home range and movement patterns of Amazonian dwarf caimans may be affected by the flooding regime in these wetland systems.
By radio-tracking for over three years sympatric adult P. palpebrosus and P. trigonatus males in a floodplain-stream system of the Amazon basin, we evaluated how the seasonal hydrological regime influences habitat occupancy, movements, home-range size and individual location within the studied streams.
Materials and methods
Study site
The study was carried out between 18 May 2014 and 18 July 2017 along the shores of two streams () located on the lower portion of the Purus River, both directly influenced by the flood pulse. We tracked five P. palpebrosus and seven P. trigonatus individuals using VHS radio transmitters. All tracked individuals were sub-adults or adults and no hatchlings were monitored in this study. The streams belong to two different floodplain-stream watersheds with similar physical characteristics [Citation24]. During the low-water season (normally between September and January) these are small floodplain streams. However, during the high-water season (between April and July), the Purus River inundates the entire watershed and water level can rise over 10 m.
Figure 1. Study area showing (a) Amazon basin (black outline) and main-rivers network (light blue) and Purus river (dark blue); (b) Streams (S#1 and S#2; blue line) where caimans were monitored are affluents of the Purus River.
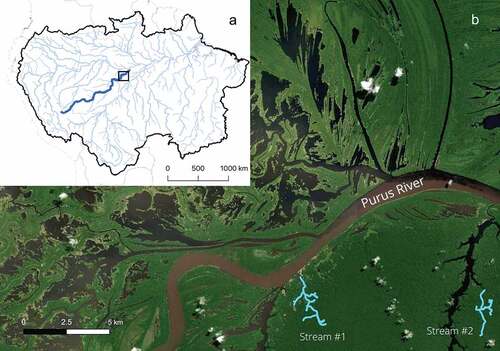
Caimans in this area are not hunted or otherwise persecuted by humans in either of the studied localities, although local residents commonly use both areas for subsistence fishing or family agriculture activities.
Variation in the water level of Purus River (in cm) was collected at a nearby scientific station of the Instituto Piagaçu, and was calibrated with the river levels reported by the Brazilian National Water Agency (www.ana.com.br). The Purus River annual flood pulse is considered the most predictable of 90 rivers worldwide [Citation27] and its mean annual amplitude was 1002 cm during the past 20 years [Citation24]. During the three-year study period, maximum river-level amplitude was 1357 cm.
Telemetry
Tracked individuals were captured on different dates and always at night during the high-water season () with locking cable snares on a restraining pole. The following morning at the nearby scientific station located <10 km from the study sites, we sexed, measured and weighed the caimans (snout-vent length [SVL] in cm, total length [TL] in cm and mass in kg), then attached to each caiman a VHF radio transmitter (Advanced Telemetry System®, Minnesota-US: Model A2930B, 65 g to 75 g weight) with frequency between 152 and 165 MHz and battery life of approximately 400 days. The transmitter was attached to the dorsal side of the tail between double scutes () just proximal to the first single scute [Citation28] as described in Brien and Read [Citation29]. We did not use anaesthesia because of possible long recovery times, prioritizing returning the animal to the capture site soon after procedure [Citation30]. The maximum time between capture and release was 12 h, which occurred at the same point. All procedures described were carried out under authorizations emitted by the Brazilian Environmental Ministry (MMA license number 53,343–3), and by the Committee for Animal Use Ethics (CEUA/INPA), registered with protocol n° 033/2017, SEI 01280.000772/2017-61.
Table 1. Summary data for radio-tracked males Paleosuchus trigonatus (PT) and Paleosuchus palpebrosus (PP). Individual identification (id), species (sp), stream captured (str), snout-vent length in cm (svl), tracking start and end dates (date_start; date_end), total number of locations (total loc), locations with intervals less than 7 days (loc <7 days), total cumulative distance moved in meters (cumul), mean daily movement in meters (avg mov_day) and percentage of movements larger than 100 m per day (mov_day > 100)
The number of caimans tracked per week during the 155-week study, varied from two to nine individuals and monitoring was carried out between 07:00 and 17:30 during the day with few exceptions (<5%) when some locations were registered during night surveys in the same locations. Tracking was carried out from a small canoe at least twice a week during the high-water seasons. However, during low-water seasons, tracking frequency was reduced due to inaccessibility of sampling sites. For both species, more than 70% of total locations were monitored with a maximum of four-day intervals. However, in 13.5% of cases for P. palpebrosus and 15.3% for P. trigonatus, the interval between locations was longer than seven days. The latter locations were not considered for daily-movement analysis because the long interval could affect results [Citation25].
Once an individual was located, we recorded its GPS position using a Garmin 78S GPS receiver, always with at least five meters accuracy. In each location, we gathered information on stream depth and width, and caiman distance to the land-water interface. We then calculated the distance of each location to the larger-stream main channel using satellite images.
Data analysis
Caiman movements were considered as the distance moved per day [Citation31] and estimated as daily movements (mov/day = distance between consecutives locations/number of days). We also estimated cumulative distance as equivalent to the sum of distances between all locations (). In order to estimate location of all caimans within their respective home range, we considered the most downstream position (MDP) of every tracked individual as a reference point.
With sufficient locations (>35), the area traversed by an individual can be described as a probability function [Citation32] and we estimated utilization distributions considered as individual home ranges [Citation33] using fixed-kernel densities (KDE). Using the bandwidth estimator with least square cross-validation (h-lscv) resulted in highly patchy areas [Citation26], so we used only the reference bandwidth (h-ref = 114 m) to estimate home-range sizes. The choice of bandwidth is critical to estimate the positions of outer contours of utilization distributions [Citation29,Citation34,Citation35]. We considered 95% contours to be total home ranges for individual caimans and 50% contour areas to be core-use areas as commonly used with telemetry studies [Citation36–38].
The Minimum Convex Polygon (MCP) has been frequently used with crocodilians [Citation25,Citation30,Citation39,Citation40], but it has many limitations and several publications have concluded that this method might overestimate the area that animals normally use because it can be strongly influenced by outliers [Citation41,Citation42]. Nevertheless, it permits evaluation of home range with less locations per animal and is simpler to estimate [Citation43], so for comparisons between seasons (high- and low-water) we considered MCP polygons only with points corresponding to each season as the area used by individuals. These results must be interpreted cautiously as home range size did not stabilize due to the low number of fixes for some individuals, especially for seasonal estimation, and this increases uncertainty [Citation29].
Distance between locations, area calculation and other GIS analyses were undertaken using the QGIS 3.4 Madeira package [Citation44]. We used the R 3.6 [Citation45] adehabitatHR package [Citation46] to estimate home-range sizes (hectares – ha) and the nlme package [Citation47] for maximum-likelihood linear mixed models described in results (LME). Independent-sample t-tests were used to compare species home-range sizes and individual movement rates. Figures were produced using the ggplot2 package also in R software [Citation48].
Results
We installed VHF radio-transmitters on three male P. trigonatus individuals in stream #1 (−61.8041 W; −4.3020 S at the mouth, ) and four males in stream #2 (−61.7111 W; −4.3044 S, ). Transmitters were also installed on five male P. palpebrosus only in stream #1 (). Snout-vent length of P. palpebrosus varied from 55.1 to 97.4 cm (mean 78.3 cm ± 16) and 80% of individuals were considered adults [Citation49]. For P. trigonatus individuals, SVL varied from 44.4 to 89.0 cm (mean 65.8 cm ± 15). We assumed that 57% of individuals were adults [supposing a minimum reproductive body size of SVL > 65 cm for males; [Citation50]. More detailed information about tracked caimans is summarized in . Mean SVL did not differ statistically between species (t-test, p = 0.207), indicating that our study focused on similar size distributions for both species.
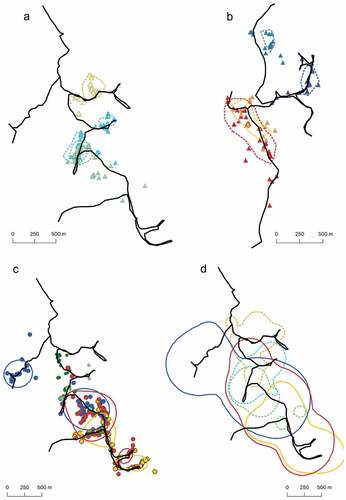
Figure 3. Locations of three male Paleosuchus trigonatus in Stream #1 (a), four males in Stream #2 (b) and five male Paleosuchus palpebrosus in Stream #1 (c); dotted lines represent core areas of each individual. Total home range overlap (d) for three males of P. trigonatus (dotted lines) and three males of P. palpebrosus (unbroken lines) estimated in Stream #1. Each colour represents an individual.
Dwarf caiman movements
Daily movements of tracked caimans varied considerably among individuals during the monitoring period, from no movement (<1% of observations) to over 200 m in a single day (4% of locations). Mean daily movements differed between species (t-test, p = 0.015); P. palpebrosus were more active (mean = 78 m/day ± 25) than P. trigonatus (mean = 36 m/day ± 13). All five P. palpebrosus males showed daily movements over 100 m/day (), which represented 26% of their moves. Four P. trigonatus were never recorded moving 100 m/day and the other three males moved that distance only in 13% of cases.
Results () of a linear mixed-effect (LME) regression model (daily movement ~ water level:species + body size:species, random|id) provided strong evidence that water level influenced movements of P. palpebrosus (p < 0.001), but the evidence was weaker for P. trigonatus (p = 0.066). Individual body size affected movements of P. trigonatus (p = 0.025) but had no detectable effect on P. palpebrosus (p = 0.135). Water level influenced mean distance moved daily by both species, principally after the dry season () when water level was rising.
Figure 4. Variation in daily mean movement of (a) Paleosuchus palpebrosus (circles) and (b) Paleosuchus trigonatus (triangle) as a function of main-river water level during three periods: low water (white), transition (black) and high water (dark grey). Each point represents an individual location.
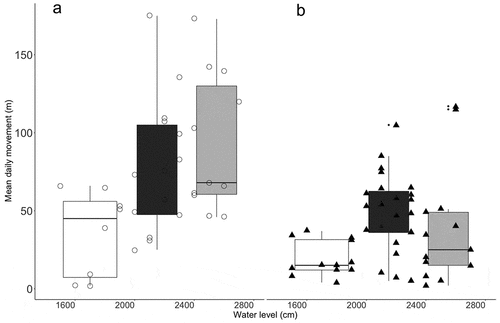
Table 2. Linear mixed-effect model (mov_day ~ water level:sp + size:sp ~ 1|id) showing the influence of main-river water level (WL) and body size on Paleosuchus palpebrosus (pp) and Paleosuchus trigonatus (pt) daily movements. The id term was a random effect, the rest were fixed
Home-range locations
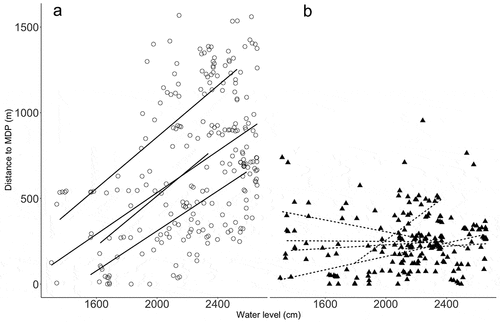
The position of individuals within the stream drainage varied as a function of the main-river water level and the depth of the stream, which varied from zero to 8.6 m (mean = 3.7 m ± 2.2) at caiman locations. The mean distance that individuals moved away from their respective most downstream position (MDP) differed between species (t-test; p < 0.001; mean 700 m in P. palpebrosus and 249 m in P. trigonatus). A LME regression model (distance to MDP ~ water level:species + size:species, random|id) indicated a positive effect of water level on the distance that individuals moved from their MDP in P. palpebrosus (p < 0.001), but the evidence was weaker for P. trigonatus (p = 0.066). Body size significantly affected distance to MDP in P. trigonatus (p = 0.034), but not for P. palpebrosus individuals (p = 0.877). Nevertheless, individual P. trigonatus responded idiosyncratically to water level rise, some moving upstream, and others returning to MDP ().
Figure 5. Effect of main-river water level on the distance to most downstream position (MDP – m) of A) Paleosuchus palpebrosus (circles) and B) Paleosuchus trigonatus (triangles). Lines represent linear-regression models for each male P. palpebrosus (black lines) or P. trigonatus (dotted lines).
Stream depth was positively related to the Purus River water level (Pearson r = 0.71) and all tracked caimans remained close to the principal stream channel with permanent water during the four months of the low-water season. Individual P. palpebrosus were encountered 35% of the time in the stream channel and moved on land at distances up to 32 m from the water. In contrast, P. trigonatus were encountered in 70% of the cases in the stream channel, and no individual moved farther than 5.5 m from main channel.
During the high-water-level season, all individuals moved farther away from the main channel into the flooded forest and there was some evidence of a difference between species (t-test; p = 0.065). Individual P. palpebrosus were located 70% of the time at more than 10 m away from the stream and some moved longer distances away from the middle of the main channel (maximum = 245 m, mean = 31 m). In contrast, 43% of locations of P. trigonatus were less than 10 m from the main channel (maximum = 226 m mean = 25 m).
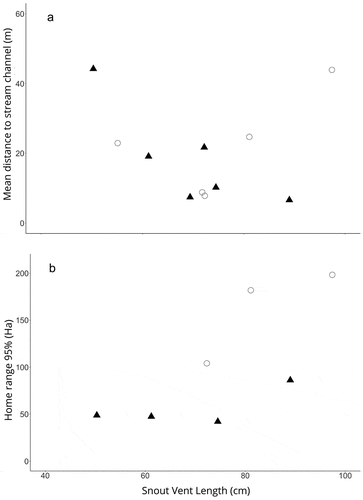
A LME regression model (distance to stream channel ~ depth + species + size + size*sp, random|id) indicated that the distance a caiman moved away from the main channel was affected only by stream depth (p < 0.001) but not by the species (p = 0.341) or individual body size (p = 0.410). The model also indicated that there was no interaction between size and species (p = 0.289). A simple linear regression model () was consistent with the conclusion that body size did not influence the distance P. palpebrosus moved from the main channel (p = 0.255, r2 = 0.19), but indicated that larger P. trigonatus moved away from the main channel less than smaller caimans (distance to stream channel ~ size; p = 0.043, r2 = 0.60), so the lack of significance of the overall model should be interpreted cautiously.
Figure 6. Relationship between snout-vent length (cm) and (a) mean distance (m) individuals moved away from the main stream channel and (b) entire home-range size (ha) estimated using Kernel Density Estimates for Paleosuchus palpebrosus (circles) and Paleosuchus trigonatus (triangles). Each point represents an individual.
Home-range areas
All 12 males (seven P. trigonatus and five P. palpebrosus) had their total MCP estimated. However, data from two individuals (one of each species) were eliminated because of insufficient locations to estimate MCP during both low- and high-water seasons, respectively (). Total home range of P. trigonatus varied from 5.0 to 30.1 ha (mean = 17.8 ha ± 9.0) and home range of P. palpebrosus varied from 35.0 to 88.6 ha (mean = 60.7 ha ± 21.9).
Table 3. Estimated home-range size in hectares for seven male (id) Paleosuchus trigonatus (sp-PT) and five male Paleosuchus palpebrosus (sp-PP). href95 = Kernel density estimator with 95% probability corresponding to entire area of use, href50 = Kernel density estimator with 50% probability and corresponding to core area, mcp100 = Minimum convex polygon with 100% of points registered, mcp_high = Minimum convex polygon with 100% of points registered in the high-water season, mcp_low = Minimum convex polygon with 100% of points registered in the low-water season. Individuals without estimated area did not have sufficient locations for that estimator
Only seven individuals (three P. palpebrosus and four P. trigonatus) had the minimum number of locations needed to estimate both the entire and the core home-range areas using the kernel-density estimator (). Kernel home ranges were greater than those estimated with MCP for both species. For P. palpebrosus individuals, it varied from 104.1 to 198.3 ha (mean = 161.4 ha ± 50.3), and core areas varied between 26.1 and 45.3 ha (). P. trigonatus entire home ranges were generally smaller (t-test, p = 0.055), varying from 42.1 to 86.2 ha (mean = 56.2 ha ± 20.2), and core areas varied from 6.5 to 20.7 ha (). All adult P. palpebrosus showed two distinct core areas, with centroids that were always more than 650 m from each other. In contrast, only one P. trigonatus showed two core areas; all other individuals had only one core area.
Considering all tracked individuals, a multiple linear regression model (home range ~ body size + species; p = 0.018, R2 = 0.80, df = 4) indicated that the size of the entire home range was influenced by the species (p = 0.033) but not by their body size (p = 0.139). This was consistent with simple linear regressions that did not detect effects of male size on home-range size for either P. palpebrosus (p = 0.338) or P. trigonatus (p = 0.283; ). Similarly, there was no detectable effect of individual size on core-area size for P. palpebrosus or P. trigonatus (p = 0.466 and p = 0.257, respectively).
Home-range overlap
We analysed the data for the six males tracked in the same stream (three for each species) to quantify their inter- and intra-specific overlap in home ranges estimated with the kernel estimator (). Home ranges of most tracked individuals overlapped, except for the smallest P. trigonatus (SVL = 50.3 cm) and the smallest P. palpebrosus (SVL = 72.3 cm). Those individuals did not overlap their home ranges because they used different portions of the stream. Overlap of home ranges for P. palpebrosus individuals was similar intra- (mean = 55%) and inter-specifically (mean = 52%). In contrast, the intraspecific overlap of entire home ranges in P. trigonatus was lower (mean = 32%) than that of P. palpebrosus (). This was more evident at the interspecific level, where the overlap was always less than 20% (mean = 13%). The extent of intraspecific-overlap areas within core areas of P. palpebrosus was double (41%) that of P. trigonatus (22%). Furthermore, the mean probability (0.62) of a P. palpebrosus individual overlapping the area of a P. trigonatus individual was almost twice that of the probability of a P. trigonatus (0.33) overlapping a P. palpebrosus area.
Table 4. Mean percentage (minimum and maximum) area overlap for three male Paleosuchus palpebrosus and three male Paleosuchus trigonatus co-existing in stream #1. over_same: mean percentage overlap over entire home range (95) and core area (50) of individuals of the same species; over_diff: mean percentage overlap over entire home range (95) and core area (50) for individuals of the other species
Discussion
Little is known on the spatial ecology Paleosuchus trigonatus and Paleosuchus palpebrosus, two of the most cryptic and understudied vertebrate predators in the Amazon basin, and there is no published information on home-range size and habitat occupancy in areas under the influence of seasonal floods.
Overall, our results show that P. palpebrosus individuals move greater distances on a daily basis and have larger home ranges than P. trigonatus. Additionally, both species show distinct behaviors when water level rises. Thus, presumably habitat occupancy patterns as a function of water-level variation facilitates coexistence of the two species of dwarf caimans.
Phylogenetically closely related species with similar morphological characteristics, such as dwarf caimans [Citation51], could be highly competitive due to niche conservatism [Citation52]. However, we might expect syntopic species to exhibit differences in resource use that would reduce competition. Many sympatric crocodilian species show different snout shapes [Citation8,Citation53,Citation54], as observed in Paleosuchus species [Citation55]. These morphological differences usually evolved with divergences in diet and feeding behavior [Citation56]. Differences in resources consumed or the way that these are obtained reduce interspecific competition [Citation57], as observed in sympatric neotropical tree boas exhibiting food-resource partitioning [Citation58].
Such differences could also be related to spatial segregation facilitated by morphological differences and habitat occupancy, a common pattern in small stream fish [Citation59], which compose an important part of the diet of some caiman [Citation13]. In the case of Paleosuchus species, additional to divergences in dependence on terrestrial or aquatic prey previously found in the same region [Citation15], the differences in habitat occupancy found in this study could be a factor that further facilitates their coexistence. When water levels rise, all P. palpebrosus moved principally upstream toward new aquatic environments formed in flooded forests. This behavior is similar to that of their main prey, as most fish species of floodplain areas occupy flooded forests when water levels rise [Citation60] to take advantage of the temporally abundant resources. In contrast, P. trigonatus individuals, who are less dependent on aquatic prey [Citation13,Citation15], behaved differently as a function of water-level variation. Their movements seem more complex and less predictable, as some individuals moved upstream, and others moved downstream, as has been observed in the Madeira River [Citation37] and French Guiana [Citation21].
Throughout the Amazon basin, animal species living in floodplains near major rivers are subjected to seasonal floods and droughts, and thus they must be ecologically adapted to radical changes in these habitats [Citation24]. The main effects of water level on the ecology of Amazonian crocodilians are related to habitat utilization [Citation8,Citation20], changes in population density [Citation11], availability of nesting sites [Citation18], or variation in diet throughout the year [Citation14]. Our results indicate that movements and spatial position within home ranges of Paleosuchus species are also influenced by seasonal water-level variation, as reported for other caiman species in a different environment [Citation25].
During the low-water season, home-range area and daily movements of both species decrease. During the low-water season, both species show average home ranges about half the size of the total area occupied. This has been observed in other crocodilian species [Citation29,Citation30]. Some individuals were occasionally found on land during the low-water season. Larger P. trigonatus individuals usually remained near the main stream channel and were relatively sedentary as reported in previous studies [Citation21,Citation50]. This is in line with findings from small non-flooded forest streams, where hatchlings disperse over large distances, but adults do not, usually remaining in burrows or retreats near the stream bank [Citation50]. This behavior has also been reported in P. palpebrosus [Citation61] and the African dwarf crocodile (Osteolaemus tetraspis) [Citation62] and may explain why transmitter signals were weak and occasionally some individuals were often not located.
Intraspecific home-range overlap was higher than interspecific overlap in both dwarf caiman species indicating a major interaction with individuals of their own species. Home-range overlap by P. trigonatus conspecifics was lower than the overlap observed in one non-seasonally flooded forest stream [Citation21] and similar to that found in a previous study [Citation50], both suggesting that males are territorial.
In our study area, P. palpebrosus showed higher mobility and the median home-range size was twice that of P. trigonatus. In the floodplains of the mid-reaches of the Madeira River, the median home-range size for P. palpebrosus was only 25% greater than that of P. trigonatus [Citation37]. However, water-level variation in our study site was 6.5 m greater than that in the Madeira River site and the greater flooding intensity in our study area might reduce available areas for P. trigonatus, whose home-range size was half that reported for the Madeira River site [Citation37].
Both species of Paleosuchus are relatively small [Citation13,Citation49], they can occur in the same locality [Citation12,Citation37,Citation63,Citation64] and sometimes use the same type of habitat, such as steep banks [Citation20] or small streams with low temperatures [Citation65,Citation66]. However, this study shows that even when living in sympatry under a seasonal flooding regime, the two Paleosuchus species show a degree of habitat segregation based on different daily movement rates, distinct home-range sizes and home-range centroids. Habitat use by these two cryptic species in the study area is complex and further studies in more localities, including hatchlings, juveniles and adult females, should be undertaken to fully understand the processes that lead to their coexistence.
Geo-location information
The study was carried out along the shores of two streams (; stream #1: −61.8041 W; −4.3020 S at the mouth, and stream #2: −61.7111 W; −4.3044 S), directly influenced by the flood pulse of the lower Purus River (Brazil), a major affluent of the Amazon River.
Authors contributions
BM: study design, fieldwork, data analysis and writing. WEM: study design, data analysis and writing. RCV: writing. FV: fieldwork, data analysis and writing.
Acknowledgments
The authors thank Eliton Miranda and Mario Jorge Bastos for their essential help in the field; without their contribution this study would not have been possible. Instituto Piagaçu provided logistic support for the field base in the Purus River. The Instituto de Desenvolvimento Sustentável Mamirauá and the National Council for Scientific and Technological Development (CNPq) provided financial support for this study (Project 449611/2014-0). The Brazilian environmental authorities provided research permits (nb 53343-1) and the study was carried out following animal-ethics permit nb 033/2017, SEI 01280.000772/2017-61 from the Instituto Nacional de Pesquisas da Amazonia (CEUA-INPA). BM received a doctoral scholarship from CNPq. WEM was supported by CNPq, the Program for Biodiversity Research (PPBio-AmOc) and the National Institute for Amazonian Biodiversity (INCT-CENBAM). We acknowledge two anonymous reviewers for their precious insights on previous version of manuscript. We thank Carolina Bertsch for help with Figures. José António L. Barão-Nóbrega and Francesco Paolo Caputo provided useful suggestions on previous versions of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hutchinson GE. Homage to Santa Rosalia or why are there so many kinds of animals? Am Natur. 1959;93:145–159.
- MacArthur RH. Population ecology of some warblers of northeastern coniferous forests. Ecology. 1958;39:599–619.
- Griffin JN, Silliman BR. Resource partitioning and why it matters. Nat Educ Knowledge. 2011;3(10):49.
- Grigg G, Kirshner D. Biology and evolution of crocodylians. Australia: Csiro Publishing; 2015.
- Somaweera R, Nifong J, Rosenblatt A, et al. The ecological importance of crocodylians: towards evidence-based justification for their conservation. Biol Rev. 2020;95(2):936–959.
- Choudhary S, Choudhury BC, Gopi GV. Spatio-temporal partitioning between two sympatric crocodilians (Gavialis gangeticus & Crocodylus palustris) in Katarniaghat Wildlife Sanctuary, India. Aquatic Conservation: Marine and Freshwater Ecosystems. 2018;28(5):1067–1076.
- Herron JC. Body size, spatial distribution, and microhabitat use in the caimans, Melanosuchus Niger and Caiman crocodilus, in a Peruvian lake. J Herpetol. 1994;28(4):508–513.
- Ouboter PE. Ecological studies on crocodilians in Suriname: niche segregation and competition in three predators. Amsterdam Netherlands: SPB Academic Publishing; 1996.
- Rebêlo GH, Lugli L. Distribution and abundance of four caiman species (Crocodylia: alligatoridae) in Jaú National Park, Amazonas, Brazil. Revista de Biología Tropical. 2001;49:1096–1109.
- Aguilera X, Coronel JS, Oberdorff T, et al. Distribution patterns, population status and conservation of Melanosuchus Niger and Caiman yacare (Crocodylia, Alligatoridae) in oxbow lakes of the Ichilo river floodplain, Bolivia. Revista de Biología Tropical. 2008;56(2):909–929.
- Da Silveira R, Magnusson WE, Thorbjarnarson JB. Factors affecting the number of caimans seen during spotlight surveys in the Mamirauá Reserve, Brazilian Amazonia. Copeia. 2008;2008(2):425–430.
- Marioni B, Dutra-Araujo D, Villamarín F, et al. Amazonian encounters with four crocodilian species in one single night! IUCN/SSC. Crocodile Specialist Group Newsletter. 2013;32(4):10–13.
- Magnusson WE, da Silva EV, Lima AP. Diets of Amazonian crocodilians. J Herpetol. 1987;21(2):85–95.
- Da Silveira R, Magnusson WE, Campos Z. Monitoring the distribution, abundance and breeding areas of Caiman crocodilus crocodilus and Melanosuchus Niger in the Anavilhanas Archipelago, Central Amazonia, Brazil. J Herpetol. 1997;31(4):514–520.
- Villamarín F, Jardine TD, Bunn SE, et al. Opportunistic top predators partition food resources in a tropical freshwater ecosystem. Freshwater Biol. 2017;62(8):1389–1400.
- Marioni B, Da Silveira R, Magnusson WE, et al. Feeding behavior of two sympatric caiman species, Melanosuchus Niger and Caiman crocodilus, in the Brazilian Amazon. J Herpetol. 2008;42(4):768–773.
- Villamarín F, Marioni B, Thorbjarnarson JB, et al. Conservation and management implications of nest-site selection of the sympatric crocodilians Melanosuchus Niger and Caiman crocodilus in Central Amazonia, Brazil. Biol Conserv. 2011;144(2):913–919.
- Banon GPR, Arraut EM, Villamarín F, et al. A review on crocodilian nesting habitats and their characterisation via remote sensing. Amphibia-Reptilia. 2019;40(4):1–21.
- Barão-Nóbrega JAL, Marioni B, Botero-Arias R, et al. The metabolic cost of nesting: body condition and blood parameters of Caiman crocodilus and Melanosuchus Niger in Central Amazonia. J Comp Physiol B. 2017;188(1):127–140.
- Magnusson WE. Habitat selection, parasites and injuries in Amazonian crocodilians. Amazoniana. 1985;9(2):193–204.
- Lemaire J, Marquis O, Gaucher P. Habitat use and behaviour of Schneider’s dwarf caiman (Paleosuchus trigonatus Schneider 1801) in the Nouragues reserve. French Guiana. IUCN/SSC Crocodile Specialist Group Newsletter. 2018;40:297–315.
- Müller J, Irion G, Nunes de Mello J, et al. Hydrological changes of the Amazon during the last glacial-interglacial cycle in Central Amazonia (Brazil). Naturwissenschaften. 1995 1995 May 01;82(5):232–235.
- Irion G, de Mello JASN, Morais J, et al. Development of the Amazon valley during the Middle to Late Quaternary: sedimentological and climatological observations. In: Junk WJ, Piedade MTF, Wittmann F, et al. editors. Amazonian floodplain forests: ecophysiology, biodiversity and sustainable management. Heidelberg Germany: Ecological Studies, Springer.; 2010. p. 27–42.
- Junk WJ, Piedade MTF, Schöngart J, et al. A classification of major naturally-occurring Amazonian lowland wetlands. Wetlands. 2011 2011 Aug 01;31(4):623–640.
- Campos Z, Coutinho M, Mourão G, et al. Long distance movements by Caiman crocodilus yacare: implications for management of the species in the Brazilian Pantanal. Herpetological J. 2006;16(2):123–132.
- Campbell HA, Dwyer RG, Irwin TR, et al. Home range utilisation and long-range movement of estuarine crocodiles during the breeding and nesting season. PloS ONE. 2013;8(5):e62127.
- Jardine TD, Bond NR, Burford MA, et al. Does flood rhythm drive ecosystem responses in tropical riverscapes? Ecology. 2015;96(3):684–692.
- Da Silveira R, Amaral JV, Magnusson WE, et al. Melanosuchus Niger (Black Caiman). Long distance movement. Herpetological Rev. 2011;42(3):424–425.
- Brien ML, Read MA, McCallum HI, et al. Home range and movements of radio-tracked estuarine crocodiles (Crocodylus porosus) within a non-tidal waterhole. Wildl Res. 2008;35(2):140–149.
- Kay WR. Movements and home ranges of radio-tracked Crocodylus porosus in the Cambridge Gulf region of Western Australia. Wildl Res. 2004;31(5):495–508.
- Millspaugh J, Marzluff JM. Radio tracking and animal populations. San Diego: Academic Press; 2001.
- Worton BJ. Kernel methods for estimating the utilization distribution in home-range studies. Ecology. 1989;70(1):164–168.
- Fieberg J, Kochanny CO. Quantifying home-range overlap: the importance of the utilization distribution. J Wildl Manage. 2005;69(4):1346–1359.
- Kie JG, Matthiopoulos J, Fieberg J, et al. The home-range concept: are traditional estimators still relevant with modern telemetry technology? Philos Trans R Soc B. 2010;365(1550):2221–2231.
- Signer J, Balkenhol N, Ditmer M, et al. Does estimator choice influence our ability to detect changes in home-range size? Anim Biotelem. 2015 2015 July 01;3(1):16.
- Fujisaki I, Hart KM, Mazzotti FJ, et al. Home range and movements of American alligators (Alligator mississippiensis) in an estuary habitat. Anim Biotelem. 2014 2014 May 19;2(1):8.
- Campos Z, Mourão G, Magnusson WE. The effect of dam construction on the movement of dwarf caimans, Paleosuchus trigonatus and Paleosuchus palpebrosus, in Brazilian Amazonia. PloS ONE. 2017;12(11):e0188508.
- Caut S, Francois V, Bacques M, et al. The dark side of the black caiman: shedding light on species dietary ecology and movement in Agami Pond, French Guiana. PloS ONE. 2019;14(6):e0217239.
- Joanen T, McNease L. A telemetric study of nesting female alligators on Rockefeller Refuge, Louisiana. Proceedings of the Annual Conference of the Southeastern Association of Game and Fish Commissioners; Knoxville, TN. 1970;24:175–193.
- Bonke R, Ihlow F, Böhme W, et al. Movement patterns of Tomistoma schlegelii in the Sekonyer Kanan River (Tanjung Puting National Park, Central Kalimantan, Indonesia): preliminary range size estimates. Salamandra. 2014;50(1):40–52.
- Worton BJ. Using monte carlo simulation to evaluate kernel-based home range estimators. J Wildl Manage. 1995;59(4):794–800.
- Nilsen EB, Pedersen S, Linnell JDC. Can minimum convex polygon home ranges be used to draw biologically meaningful conclusions?Ecol Res. 2008 2008 May 01;23(3):635–639.
- Huck M, Davison J, Roper TJ. Comparison of two sampling protocols and four home-range estimators using radio-tracking data from urban badgers Meles meles. Wildl Biol. 2008;14(4):467–477, 11.
- QGIS Development Team. QGIS geographic information system. Open Source Geospatial Foundation Project. 2019.
- R Development Core Team. R: a language and environment for statistical computing. Vienna Austria: R Foundation for Statistical Computing; 2019.
- Calenge C. The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals.Ecol Modell. 2006 2006 Aug 25;197(3):516–519.
- Pinheiro J, Bates D, DebRoy S, et al. nlme: linear and nonlinear mixed effects models. R Package Version 3.1. 2020;3:147. doi:10.1007/b98882.
- Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag; 2016.
- Campos Z, Magnusson WE, Marques V. Growth rates of Paleosuchus palpebrosus at the Southern Limit of its range. Herpetologica. 2013;69(4):405–410.
- Magnusson WE, Lima AP. The ecology of a cryptic predator, Paleosuchus tigonatus, in a tropical rainforest. J Herpetol. 1991;25(1):41–48.
- Muniz FL, Ximenes AM, Bittencourt PS, et al. Detecting population structure of Paleosuchus trigonatus (Alligatoridae: caimaninae) through microsatellites markers developed by next generation sequencing. Mol Biol Rep. 2019;46(2):2473–2484.
- Wiens JJ, Ackerly DD, Allen AP, et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol Lett. 2010;13(10):1310–1324.
- Pearcy A. Implications of skull shape for the ecology and conservation biology of crocodiles. Leiden Netherlands: Leiden University; 2011.
- Brochu CA. Crocodylian snouts in space and time: phylogenetic approaches toward adaptive radiation. Am Zool. 2001;41:564–585.
- Medem F. Los Crocodylia de sur América. Bogotá Colombia: Colciencias; 1983.
- Walmsley CW, Smits PD, Quayle MR, et al. Why the long face? The mechanics of mandibular symphysis proportions in crocodiles. PloS ONE. 2013 Jan 16;8(1):e53873.
- Chesson P. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst. 2000;31(1):343–366.
- Henderson RW. Neotropical treeboas: natural history of the Corallus hortulanus complex. Melbourne FL: Krieger Publisher; 2002. 1575240386.
- Silva NCDS, Costa AJLD, Louvise J, et al. Resource partitioning and ecomorphological variation in two syntopic species of Lebiasinidae (Characiformes) in an Amazonian stream. Acta Amazonica. 2016;46:25–36.
- Stegmann LF, Leitão RP, Zuanon J, et al. Distance to large rivers affects fish diversity patterns in highly dynamic streams of Central Amazonia. PLoS ONE. 2019;14(10):e0223880.
- Roberto IJ, Albano CG. Paleosuchus palpebrosus (Cuvier’s Smooth-Fronted Caiman). Habitat Use. Herpetolofical Review. 2014;45(1):121–122.
- Shirley MH, Burtner B, Oslisly R, et al. Diet and body condition of cave-dwelling dwarf crocodiles (Osteolaemus tetraspis, Cope 1861) in Gabon. Afr J Ecol. 2017;55(4):411–422.
- Medem F. Los Crocodylia de sur América. Vols. 1-2. Bogotá Colombia: Ministerio de Educación Nacional, Fondo Colombiano de Investigaciones Científicas y Proyectos Especiales; 1981.
- Lugo M, Lasso CA, Castro A, et al. Paleosuchus trigonatus (Schneider 1801). In: Morales-Betancourt MA, Lasso CA, De La Ossa J, et al. editors. Biología y Conservación de los Crocodylia de Colombia - Serie Recursos Hidrobiolgógicos y Pesqueros Continentales de Colombia. Serie Recursos Hidrobiolgógicos y Pesqueros Continentales de Colombia. Vol. VIII, Bogotá D. C. Colombia: Instituto de Investigación de Recursos Biológicos Alexander von Humboldt (IAvH); 2013. p. 201–210.
- Magnusson WE, Lima AP, Hero J-M, et al. Paleosuchus trigonatus nests: sources of heat and embryo sex ratios. J Herpetol. 1990;24(4):397–400.
- Campos Z, Magnusson WE. Thermal relations of dwarf caiman, Paleosuchus palpebrosus, in a hillside stream: evidence for an unusual thermal niche among crocodilians. J Therm Biol. 2013;38(1):20–23.

