Abstract
To report a case of pediatric recurrent meningitis that is associated with an underlying petrous apex encephalocele in a patient with bilateral cochlear implants and reviews the literature of similar petrous apex lesions. A 5-year-old girl presented during her third episode of meningitis. Her first episode of meningitis left her with profound bilateral sensorineural hearing loss for which she received cochlear implants. During her assessment, she was found to have a petrous apex encephalocele, which was successfully repaired via a middle cranial fossa approach. Literature review demonstrates that petrous apex cephaloceles (PAC) are associated with meningitis, particularly in children. No previous reports exist of PAC in patients with cochlear implants. This case illustrates the importance of identifying an underlying anatomic abnormality in recurrent meningitis. PAC are rare abnormalities that can cause cerebrospinal fluid leaks leading to intracranial hypotension, cranial neuropathies, and infectious complications.
Introduction
Over the past several decades, the incidence of pediatric bacterial meningitis has decreased due to the routine use of Haemophilus influenzae type b vaccination, pneumococcal conjugate vaccination, and universal screening of pregnant women for group B streptococcus. Current estimates of the incidence of bacterial meningitis among children from birth to 17 years range from 0.4 to 80.7 per 100,000 population.[Citation1] However, in spite of decreasing incidence, meningitis is still a life-threatening illness and otolaryngologists and neurosurgeons are essential to the evaluation of patients with recurrent episodes as an intracranial, spinal, or inner ear abnormality is usually the cause.[Citation2]
Sensorineural hearing loss (SNHL) is the most common major sequela of bacterial meningitis and is found in more than one-third of cases [Citation3] and may require prompt cochlear implantation for cases of severe-to-profound SNHL. Cochlear implantation has been performed in roughly 38,000 pediatric patients to date.[Citation4] While considered a safe surgical procedure, CI does carry an increased risk of infectious complications including up to a 30-fold increase in bacterial meningitis compared to the general pediatric population.[Citation5–7]
The evaluation of pediatric patients with concern for bacterial meningitis is challenging due to variability in presenting signs and symptoms.[Citation8,Citation9] This case demonstrates the multiple underlying etiologies to consider when presented with a patient with recurrent meningitis.
We performed a review of the literature of cases of petrous apex cephaloceles (PAC) – including arachnoid cysts, meningoceles, and encephaloceles – to better characterize their association with meningitis and other complications. To our knowledge no other cases of recurrent meningitis from a PAC have been reported in the setting of cochlear implantation.
Case report
A previously healthy 3-year-old girl presented to an outside medical facility with 1 year of recurrent headaches. Evaluation at that time led to a diagnosis of a Chiari type I malformation for which a posterior fossa decompressive procedure was considered. However, in September 2014 at the age of 4, she developed fever, headache, and fluctuating mental status and a clinical diagnosis of meningitis was made. She previously completed age appropriate vaccinations including pneumococcal conjugate vaccine-13. Due to a concern for an otologic source of the infection, bilateral myringotomy with tympanostomy tube placement was performed, which revealed clear fluid from the left middle ear. She was promptly treated with intravenous antibiotics and had a full recovery. Soon after discharge, she developed bilateral sudden severe-to-profound SNHL and appropriately underwent expedited bilateral cochlear implantation.
Three months after cochlear implantation, she developed fever, headache, and visual disturbance concerning for a second episode of meningitis. During this episode, she was noted to have profuse clear tube otorrhea and rhinorrhea but this was not able to be collected for β-2 transferrin testing. Cerebrospinal fluid (CSF) studies showed pleocytosis, elevated protein, and low glucose content, all consistent with bacterial meningitis. Blood cultures grew Streptococcus pneumoniae. She quickly recovered with broad spectrum antibiotics.
She re-presented in September 2015 at the age of 5 with another episode of severe headache, nausea, vomiting of clear fluid, lethargy, and neck rigidity, consistent with a third episode of meningitis. She was transferred to our medical center for further evaluation and management. Within hours of admission, she developed acute 6th and 7th cranial nerve palsies and seizures requiring intubation for airway control.
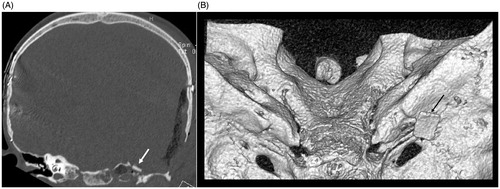
Computed tomography (CT) of the head and magnetic resonance imaging (MRI) of the brain in a 1.5 Tesla magnet were obtained which showed severe intracranial hypotension with severe sagging brain (Figure ) indicating a low CSF volume state. Imaging also showed a large left petrous apex bone defect with an associated encephalocele. The MRI images do not have characteristic magnetic artifact because magnets have been removed from the internal cochlear receiver stimulator (Figure ). CSF studies were not obtained due to intracranial hypotension. There was no clinical evidence of CSF otorrhea or rhinorrhea at the time. Peripheral blood studies showed a leukocytosis and blood cultures obtained after initiation of antibiotics grew no organisms.
Figure 1. Pre-operative sagittal T1-weighted MRI showing severe sagging of the brainstem and cerebellum (white arrow) from low CSF volume state in setting of a Chiari malformation.
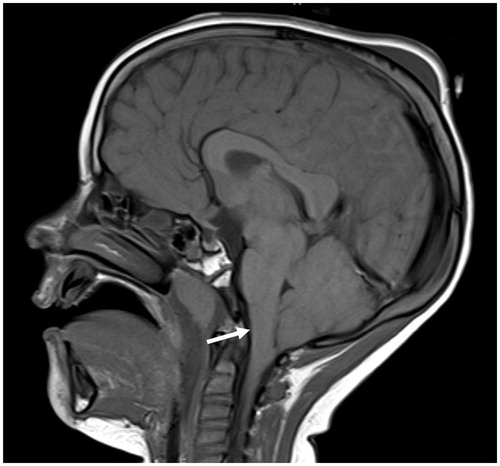
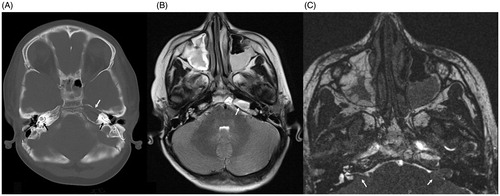
Figure 2. Pre-operative axial CT scan showing bony defect in left petrous apex filled with fluid/soft tissue density material (white arrow). Also seen are bilateral cochlear implant electrodes in place (black arrows) (A), axial T2-weighted MRI showing fluid and soft tissue in the left petrous apex (white arrow) (B), axial CISS MRI showing fluid and soft tissue in the left petrous apex. Cerebellar flocculus can be seen protruding into the internal acoustic meatus as a consequence of severe CSF hypotension (white arrows) (C). Note: MRI images do not have characteristic magnetic artifact because magnets have been removed from the internal cochlear receiver stimulator.
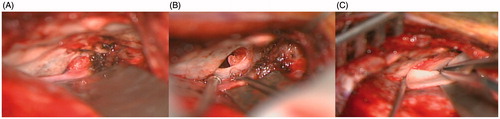
One month after completely recovering from meningitis, she was taken to the operating room for repair of the CSF leak via a left middle cranial fossa craniotomy approach to the petrous apex. A lumbar drain was inserted to obtain adequate brain relaxation. Intraoperatively she was found to have a meningoencephalocele through an approximately 1.5 × 1 cm petrous apex bone defect. The herniated brain and dura were resected and the dura was closed primarily. The petrous apex was packed with Gelfoam as support for temporalis fascia graft underlay. The defect was covered with another piece of temporalis fascia, calvarial bone graft, and synthetic dural substitute (Figure ). A post-operative CT was performed to ensure adequate repair (Figure ). She recovered well from her operation and was discharged on the fourth post-operative day. Since discharge, she has had no recurrent CSF otorhinorrhea or meningitis.
Discussion
Meningitis is a life-threatening infection and it is essential for clinicians to be comfortable with its appropriate diagnosis and management. Of particular concern to the otolaryngologist, meningitis often leads to SNHL that may require cochlear implantation and cochlear implantation itself increases the risk of meningitis.
We present a case of a pediatric patient with meningitis prior to and after cochlear implantation. During the evaluation of a patient with a history of cochlear implantation and meningitis, one must always consider the possibility of hardware infection and be ready to explant the device. However, it is just as important in patients with recurrent meningitis to perform a comprehensive evaluation including imaging of the brain, skull base, and spine since nearly all will have an identifiable anatomic abnormality, as our patient did.[Citation2] In these cases, removal of the cochlear implant hardware will not decrease the risk of recurrent meningitis and will have detrimental effects on hearing restoration.
Spontaneous encephaloceles of the temporal bone are relatively common sources of CSF leak treated by the neurotologist and neurosurgeon. However, they are most commonly identified in defects of the tegmen mastoideum and tegmen tympani.[Citation10–13] In this case, we were able to identify a petrous apex encephalocele associated with a pre-implantation CSF leak and meningitis. PACs are rare lesions that are most commonly asymptomatic but can also cause CSF leaks leading to headaches, cranial nerve palsies, and infectious complications. These lesions can be missed on imaging without careful attention beyond the surgical field and more common sites of skull base defects.
Summary of all available reports of PACs among adults and children available in the English medical literature via Medline search is presented in Table . The majority of cases (72%) have been reported in adults with a minority (28%) reported in children.[Citation14–29] 70% of the reported cases of pediatric PACs (including this report) were associated with infectious complications such as meningitis [Citation17–19,Citation30] compared to only 12% of adult cases.[Citation14,Citation15,Citation26] This may reflect an increase risk of meningitis in children or possibly difficulty of children to report other symptoms that could present before infectious complications.
Table 1. All cases of PACs in the English literature ordered by age at diagnosis.
The most frequently reported presenting symptom was headache, which was reported in 28% of cases. The most commonly impaired cranial nerve was trigeminal (39%) followed by vestibulocochlear (28%). Less frequently, patients were found to have abducens (8%), facial (6%), and oculomotor (3%) palsies.
The most common surgical approach used to repair the PAC was via a middle cranial fossa craniotomy (33%) followed by transmastoid approaches (22%). Fewer patients were treated with combined middle cranial fossa and transmastoid approaches (6%) and only one (3%) was treated via a retrosigmoid approach.
Several other groups have reported the radiographic incidence of PACs without reporting clinical histories (Table ) [Citation31–33] including one large study by Jamjoom and Alorainy who identified 111 PACs in 2410 brain MRIs leading to an estimate of 5.3% incidence of PACs in their cohort.[Citation34]
Table 2. Studies reporting of PACs without clinical data.
We describe a case of a pediatric patient with a petrous apex encephalocele leading to a CSF leak and recurrent meningitis in the setting of bilateral cochlear implantation. To our knowledge, there are no previous cases in the English literature involving a patient with a PAC and cochlear implantation. The rarity of PACs and presence of cochlear implants in this case may have led to a delay in diagnosing the PAC as the underlying source of her recurrent meningitis as previous physicians were focused on the increased risks of meningitis from the implants themselves. Similarly, at the time of her initial diagnosis of Chiari malformation the physicians missed her CSF hypotension which could have led to earlier identification of her PAC. This case highlights the importance of maintaining a high index of suspicion for other anatomical causes of meningitis, even among patients with cochlear implantation.
Conclusion
This case illustrates the importance of identifying an underlying anatomic abnormality as the source of recurrent meningitis and highlights the difficulty in doing so in children who have other risk factors for meningitis including cochlear implantation. PACs are rare abnormalities of the petrous apex that can cause CSF leaks leading to intracranial hypotension, cranial neuropathies, and infectious complications.
Funding information
Sources of Support: Departmental Funds
References
- Thigpen MC, Whitney CG, Messonnier NE, et al. Bacterial meningitis in the United States, 1998–2007. N Engl J Med. 2011;364:2016–2025.
- Lieb G, Krauss J, Collmann H, et al. Recurrent bacterial meningitis. Eur J Pediatr. 1996;155:26–30.
- Edmond K, Clark A, Korczak VS, et al. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:317–328.
- Cochlear Implants. National Institute on Deafness and Other Communication Disorders; 2014 [Internet]. Available from: http://www.nidcd.nih.gov/health/hearing/pages/coch.aspx.
- Reefhuis J, Honein MA, Whitney CG, et al. Risk of bacterial meningitis in children with cochlear implants. N Engl J Med. 2003;349:435–445.
- Reefhuis J, Whitney CG, Mann EA. A public health perspective on cochlear implants and meningitis in children. Otol Neurotol. 2010;31:1329–1330.
- Javia L, Brant J, Guidi J, et al. Infectious complications and ventilation tubes in pediatric cochlear implant recipients. Laryngoscope. 2015 Sep 7. [Epub ahead of print].
- Kim KS. Acute bacterial meningitis in infants and children. Lancet Infect Dis. 2010;10:32–42.
- Bilavsky E, Leibovitz E, Elkon-Tamir E, et al. The diagnostic accuracy of the ‘classic meningeal signs’ in children with suspected bacterial meningitis. Eur J Emerg Med. 2013;20:361–363.
- Papanikolaou V, Bibas A, Ferekidis E, et al. Idiopathic temporal bone encephalocele. Skull Base. 2007;17:311–316.
- Kapur TR, Bangash W. Tegmental and petromastoid defects in the temporal bone. J Laryngol Otol. 1986;100:1129–1132.
- Nahas Z, Tatlipinar A, Limb CJ, et al. Spontaneous meningoencephalocele of the temporal bone: clinical spectrum and presentation. Arch Otolaryngol Head Neck Surg. 2008;134:509–518.
- Pelosi S, Bederson JB, Smouha EE. Cerebrospinal fluid leaks of temporal bone origin: selection of surgical approach. Skull Base. 2010;20:253–259.
- Kou YF, Allen KP, Isaacson B. Recurrent meningitis secondary to a petrous apex meningocele. Am J Otolaryngol. 2014;35:405–407.
- Moore BC. Characterization and simulation of impaired hearing: implications for hearing aid design. Ear Hear. 1991;12:154S–161S.
- Cheung SW, Broberg TG, Jackler RK. Petrous apex arachnoid cyst: radiographic confusion with primary cholesteatoma. Am J Otol. 1995;16:690–694.
- Motojima T, Fujii K, Ishiwada N, et al. Recurrent meningitis associated with a petrous apex cephalocele. J Child Neurol. 2005;20:168–170.
- Mulcahy MM, McMenomey SO, Talbot JM, et al. Congenital encephalocele of the medial skull base. Laryngoscope. 1997;107:910–914.
- Hervey-Jumper SL, Ghori AK, Quint DJ, et al. Cerebrospinal fluid leak with recurrent meningitis following tonsillectomy. J Neurosurg Pediatr. 2010;5:302–305.
- Lin BM, Aygun N, Agrawal Y. Imaging case of the month: cystic lesions of the petrous apex: identification based on magnetic resonance imaging characteristics. Otol Neurotol. 2012;33:e75–e76.
- Warade AG, Misra BK. Petrous apex cephalocele presenting with cerebrospinal fluid rhinorrhea in an adult. J Clin Neurosci. 2016;25:155–157.
- Jakkani RK, Ragavendra KI, Karnawat A, et al. Bilateral petrous apex cephaloceles. Neurol India. 2012;60:563–564.
- Achilli V, Danesi G, Caverni L, et al. Petrous apex arachnoid cyst: a case report and review of the literature. Acta Otorhinolaryngol Ital. 2005;25:296–300.
- Fois P, Lauda L. Bilateral Meckel’s cave arachnoid cysts with extension to the petrous apex in a patient with a vestibular schwannoma. Otol Neurotol. 2011;32:e36–e37.
- Falcioni M, Caruso A, Taibah A, et al. Arachnoid cysts of the petrous apex in a patient with vestibular schwannoma. Otolaryngol Head Neck Surg. 2000;123:657–658.
- Isaacson B, Coker NJ, Vrabec JT, et al. Invasive cerebrospinal fluid cysts and cephaloceles of the petrous apex. Otol Neurotol. 2006;27:1131–1141.
- Batra A, Tripathi RP, Singh AK, et al. Petrous apex arachnoid cyst extending into Meckel’s cave. Australas Radiol. 2002;46:295–298.
- Stark TA, McKinney AM, Palmer CS, et al. Dilation of the subarachnoid spaces surrounding the cranial nerves with petrous apex cephaloceles in Usher syndrome. AJNR Am J Neuroradiol. 2009;30:434–436.
- Chang P, Fagan PA, Atlas MD, et al. Imaging destructive lesions of the petrous apex. Laryngoscope 1998;108:599–604.
- Schick B, Draf W, Kahle G, et al. Occult malformations of the skull base. Arch Otolaryngol Head Neck Surg. 1997;123:77–80.
- Hatipoğlu HG, Cetin MA, Gurses MA, et al. Petrous apex cephalocele and empty sella/arachnoid cyst coexistence: a clue for cerebrospinal fluid pressure imbalance? Diagn Interv Radiol. 2010;16:7–9.
- Cavusoglu M, Duran S, Hatipoğlu HG, et al. Petrous apex cephalocoele: contribution of coexisting intracranial pathologies to the aetiopathogenesis. Br J Radiol. 2015;88:20140721.
- Alorainy IA. Petrous apex cephalocele and empty sella: is there any relation? Eur J Radiol. 2007;62:378–384.
- Jamjoom DZ, Alorainy IA. The association between petrous apex cephalocele and empty sella. Surg Radiol Anat. 2015;37:1179–1182.