Abstract
Bisphosphonate-related osteonecrosis of the jaw (BRONJ) is a rare, serious complication of bisphosphonate therapy. We report here a 69-year-old man with a history of multiple myeloma and 5 years of adjuvant zoledronate therapy presenting with loss of light perception, proptosis, ptosis and impaired abduction of the right eye. This patient was found to have developed an unusually severe presentation of osteomyelitis of the skull base in the setting of BRONJ that ultimately led to unilateral blindness. In this extreme case of bisphosphonate-related osteonecrosis of the skull base, we reveal the importance of early identification and treatment of these lesions. This is the first description of BRONJ-induced blindness.
Introduction
Bisphosphonate-related osteonecrosis of the jaw (BRONJ) is a rare, serious complication of bisphosphonate therapy that was first described in 2003.[Citation1] BRONJ can generally be defined as the persistent (>8 weeks) presence of necrotic bone in the maxillofacial region of patients with a history of bisphosphonate therapy and without radiotherapy of the jaw.[Citation2] Although it was a key component of the original American Association of Oral and Maxillofacial Surgeons criteria (AAOMS), exposed necrotic bone is not detectable up to 24% of cases.[Citation3] The diagnostic criteria of BRONJ, however, remain in flux and its pathophysiology continues to be incompletely understood.
Patients with cancer, particularly multiple myeloma, treated with long courses of intravenous (IV) zoledronate show the highest incidence of this often treatment-refractory complication. Late stages of bisphosphonate-related osteonecrosis of the maxilla are associated with oroantral fistula formation, maxillary sinusitis and osteomyelitis. We report here an unusually severe presentation of osteomyelitis of the skull base in association with BRONJ that resulted in a patient’s complete loss of light perception.
Case report
A 69-year-old man with a history of multiple myeloma presented to our institution with new onset right eye blindness in June 2015. He had been in remission of multiple myeloma since 2009 and was on adjuvant zoledronate therapy. After 5 years of quarterly zoledronate, the patient was diagnosed with right maxillary osteonecrosis in 2014. The patient underwent debridement and pathological analysis identified necrotic bone with gram positive cocci, rods and leukocytes. Five months later the patient presented with an oroantral fistula and osteomyelitis of the right maxillary sinus. Caldwell–Luc procedure was performed. Pathological examination of the maxillary sinus biopsy showed intratrabecular necrosis and focal collections of paratrabecular neutrophils. The necrotic intratrabecular space contained clusters of gram-positive cocci and Gomori methenamine silver (GMS) stain-negative filamentous organisms, consistent with acute Actinomyces osteomyelitis. The patient remained afebrile during all described encounters.
The patient first noted dimming of vision in his right eye in March 2015. He was diagnosed with glaucoma and treated with travoprost ophthalmic solution. At his first presentation to our institution 3 months later, he demonstrated complete loss of light perception, proptosis, ptosis and limited abduction of the right eye. Fundoscopic examination of the optic nerves showed mild temporal thinning of the right nerve with no apparent pallor. Despite an absence of autonomic or sensory symptoms in the left eye, the left optic nerve showed more profound thinning on fundoscopy than the right nerve.
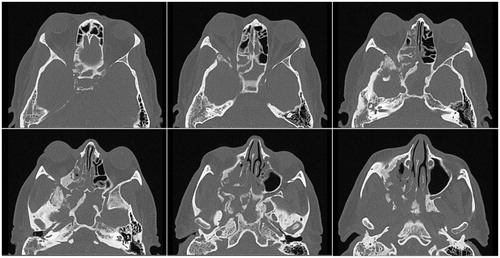
Magnetic resonance imaging (MRI) revealed a destructive lesion with involvement of the right pterygoid and sphenoid wings, right orbital soft tissue and left optic nerve atrophy (Figure ). Computerized tomography (CT) demonstrated irregular sclerosis, fragmentation and expansion of the walls of the right maxillary and sphenoid sinuses, right greater wing of the sphenoid and right pterygoid body. In addition, enlargement of the right lateral rectus muscle was observed in the inferolateral extraconal compartment of the right orbit extending posteriorly and resulting in compression of the right optic nerve near the orbital apex (Figure ). The decision was thus made to decompress the right optic nerve.
Figure 1. Preoperative coronal MRI with gadolinium contrast. MRI showing a destructive lesion with bony involvement of the right pterygoid and sphenoid wings, soft tissue enhancement of the right orbit, and left optic nerve atrophy.
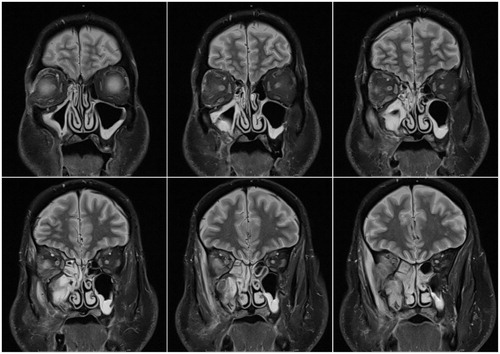
Figure 2. Preoperative computed axial tomography with contrast. CT prior to optic nerve decompression demonstrated irregular sclerosis, fragmentation and expansion of the walls of the right maxillary and sphenoid sinuses, right greater wing of the sphenoid, and right pterygoid body. In addition, enlargement of the right lateral rectus muscle was observed in the inferolateral extraconal compartment of the right orbit extending posteriorly and resulting in compression of the right optic nerve near the orbital apex.
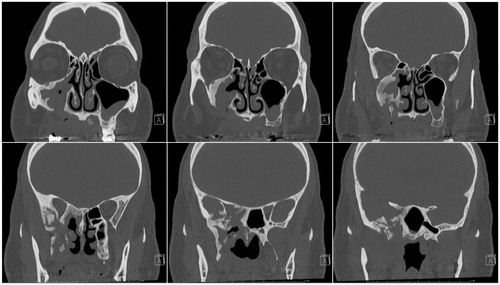
Transnasal uncinectomy and maxillary antrostomy revealed a maxillary sinus filled with chronically inflamed polypoid tissue and necrotic bone debris. Several areas of necrotic bone fragments were also found and removed from the anterior and posterior ethmoid sinuses. The posterior orbital apex was exposed to reveal a thickened lamina papyracea, which was removed. The periorbita was traced posteriorly along the optic nerve canal and followed into the sphenoid sinus. The sphenoid sinus was opened widely and several necrotic-appearing bone chips were removed. The optic nerve sheath was opened and extended anteriorly along the orbital apex through the periorbita. No injury to the carotid artery or optic nerve was noted. The optic nerve and posterior orbital contents did not appear to be under great pressure and no purulence was observed. The patient was placed on a 3-month course of amoxicillin and showed no evidence of disease progression on follow-up (Figure ). Unfortunately, he has regained minimal light perception to the affected eye.
Figure 3. Post-operative computed coronal tomography with contrast at 3 months. Follow-up CT showing unchanged irregular sclerosis, fragmentation and expansion of the walls of the right maxillary sinus, right sphenoid sinus, right greater sphenoid wing and right pterygoid body, consistent with sequela of the patient’s biopsy-proven infected osteonecrosis status post therapy. The right orbital apex soft tissues showed near-resolution of attenuation and right lateral rectus muscle showed reduced enlargement relative to pre-operative CT, consistent with a response to therapy. No evidence of disease progression was observed.
Discussion
The incidence of BRONJ varies greatly between oral and IV routes of bisphosphonate administration. A recent systematic review found the incidence of BRONJ from IV administration to be 7%, with a range of 0–27.5% over 47 prospective and retrospective studies. The overall incidence of BRONJ associated with oral administration was found to be 0.12%, ranging from 0–4.3% over nine retrospective and prospective studies.[Citation4]
A longitudinal study of 1621 cancer patients treated with IV zoledronate, ibandronate or pamidronate reported BRONJ incidences of: 8.5% in multiple myeloma, 3.1% in breast cancer and 4.9% in prostate cancer. The authors found the use of dentures (OR = 2), dental extraction (OR = 33), having ever received zoledronate (OR = 28) and each zoledronate dose (OR = 2) as significant risk factors for the development of BRONJ.[Citation5] These findings are consistent with several other independent reports.[Citation6]
The pathophysiology underlying BRONJ remains unclear and is a source of investigation. Proposed mechanisms include dysregulation of bone remodeling, inhibition of angiogenesis and local infection.[Citation7] The true mechanism of BRONJ initiation and progression is likely a result of the interplay of a combination of these factors.
Bisphosphonates reduce bone resorption through the inhibition of osteoclast function.[Citation8] The inhibition of bone resorption is exploited to slow the progression of osteoporosis and to prevent the occurrence of osteolytic metastases. However, prolonged high-dose, high-potency bisphosphonate therapy may impair the osteoclast–osteoblast balance that mediates bone healing and remodeling. The importance of osteoclast inhibition in the pathophysiology of BRONJ is supported by reports that denosumab, another anti-absorptive agent, is also associated with osteonecrosis of the jaw.[Citation9]
In addition to impacting osteoclast function, bisphosphonates inhibit angiogenesis.[Citation10] The formation and maintenance of blood vessels is mediated by angiogenic factors, including vascular endothelial growth factor (VEGF), that promote the growth, migration and differentiation of endothelial cells. Both the VEGF receptor tyrosine kinase inhibitor, sunitinib,[Citation11] and monoclonal anti-VEGF antibody, bevacizumab, have been linked to osteonecrosis of the jaw, especially in conjunction with zoledronate.[Citation12] In light of these findings, the AAOMS has recommended generalizing the nomenclature from BRONJ to medication-related osteonecrosis of the jaw (MRONJ).
Dental disease and bacterial infection have also been implicated in the pathophysiology of BRONJ. The preponderance of early BRONJ reports involved tooth extraction, which continues to be a risk factor. In these cases, periodontal disease often preceded tooth extraction.[Citation13] Actinomyces species represent the predominant organisms identified in biopsied specimens of necrotic bone of patients with BRONJ.[Citation14] Despite the strong association with Actinomyces infection, it is unclear if osteomyelitis is an early initiating factor in BRONJ or whether osteonecrosis occurs independently and is subsequently seeded by microorganisms.
Regardless of the underlying mechanism, osteomyelitis in the setting of BRONJ is associated with more advanced stages of disease that is often refractory to treatment.[Citation2] We reported an unusually severe presentation of Actinomyces osteomyelitis superimposed on BRONJ that arose in the maxilla. Despite multiple debridements, the lesion progressed to oroantral fistula formation and fulminant maxillary sinusitis that extended to the skull base and orbital apex, causing optic nerve damage and unilateral blindness.
Involvement of the skull base occurs in a rarity of cases.[Citation15] BRONJ disproportionately affects the mandible; maxillary involvement occurs in approximately one-quarter to one-third of the cases.[Citation2] When the maxilla is involved, 43–48% [Citation16,Citation17] progress to maxillary sinusitis, indicating Stage 3 disease: the most treatment-refractory stage of BRONJ.[Citation2] Purulent sinusitis is a concerning finding due to the severity of its potential complications, which include blindness, meningitis, subdural empyema and cerebral abscess.[Citation18,Citation19]
Therapeutic outcomes are optimized by conservative surgery of localized BRONJ lesions, necessitating a timely diagnosis by identification of the early signs of BRONJ. Unfortunately, early signs of maxillary BRONJ include nonspecific sinus pain, odontalgia and altered neurosensory function [Citation2] which require a high index of suspicion of BRONJ when evaluating patients on anti-resorptive and anti-angiogenic agents. Identification of oroantral fistulae should dramatically raise suspicion of active disease in the maxilla.[Citation16] Diagnosis of maxillary BRONJ should prompt computed tomographic imaging of the paranasal sinuses [Citation20] to ensure prompt therapy and potentially avoid serious sequelae.
Notes on contributors
Farhoud Faraji, is an MD/PhD medical student at St. Louis University. He has completed his PhD and continues to contribute to the medical literature as he finishes his MD program.
Jastin L. Antisdel, is a fellowship trained rhinologist. He is currently the Chair designate of the department of Oto-HNS at St. Louis University.
Joseph D. Brunworth, is an assistant professor at St. Louis University. He is double fellowship trained in Rhinology/Skull-base surgery.
References
- Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117.
- Ruggiero SL, Dodson TB, Assael LA, et al. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws-2009 update. J Oral Maxillofac Surg. 2009;67:2–12.
- Fedele S, Bedogni G, Scoletta M, et al. Up to a quarter of patients with osteonecrosis of the jaw associated with antiresorptive agents remain undiagnosed. Br J Oral Maxillofac Surg. 2015;53:13–17.
- Kuhl S, Walter C, Acham S, et al. Bisphosphonate-related osteonecrosis of the jaws-a review. Oral Oncol. 2012;48:938–947.
- Vahtsevanos K, Kyrgidis A, Verrou E, et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol. 2009;27:5356–5362.
- Thumbigere-Math V, Tu L, Huckabay S, et al. A retrospective study evaluating frequency and risk factors of osteonecrosis of the jaw in 576 cancer patients receiving intravenous bisphosphonates. Am J Clin Oncol. 2012;35:386–392.
- Mehrotra B, Ruggiero S. Bisphosphonate complications including osteonecrosis of the jaw. Hematology Am Soc Hematol Educ Program. 2006;356–360, 515.
- Roelofs AJ, Thompson K, Gordon S, et al. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12:6222s–6230s.
- Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48:3082–3092.
- Wood J, Bonjean K, Ruetz S, et al. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002;302: 1055–1061.
- Roskoski R Jr. Sunitinib: a VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem Biophys Res Commun. 2007;356:323–328.
- Estilo CL, Fornier M, Farooki A, et al. Osteonecrosis of the jaw related to bevacizumab. J Clin Oncol. 2008;26:4037–4038.
- Ficarra G, Beninati F, Rubino I, et al. Osteonecrosis of the jaws in periodontal patients with a history of bisphosphonates treatment. J Clin Periodontol. 2005;32:1123–1128.
- Dimopoulos MA, Kastritis E, Bamia C, et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol. 2009;20:117–120.
- Khan AM, Sindwani R. Bisphosphonate-related osteonecrosis of the skull base. Laryngoscope. 2009;119:449–452.
- Mast G, Otto S, Mucke T, et al. Incidence of maxillary sinusitis and oro-antral fistulae in bisphosphonate-related osteonecrosis of the jaw. J Craniomaxillofac Surg. 2012;40:568–571.
- Maurer P, Sandulescu T, Kriwalsky MS, et al. Bisphosphonate-related osteonecrosis of the maxilla and sinusitis maxillaris. Int J Oral Maxillofac Surg. 2011;40:285–291.
- Bair-Merritt MH, Shah SS, Zaoutis TE, et al. Suppurative intracranial complications of sinusitis in previously healthy children. Pediatr Infect Dis J. 2005;24:384–386.
- Perloff JR, Gannon FH, Bolger WE, et al. Bone involvement in sinusitis: an apparent pathway for the spread of disease. Laryngoscope. 2000;110:2095–2099.
- Bedogni A, Fedele S, Bedogni G, et al. Staging of osteonecrosis of the jaw requires computed tomography for accurate definition of the extent of bony disease. Br J Oral Maxillofac Surg. 2014;52:603–608.
