Introduction
Squamous cell carcinoma (SCC) represents about 90–95% of all malignant neoplasms of the oral cavity. It typically occurs in the elderly men during the fifth–eight decades of life [Citation1] and rarely occurs in the young patients under the age of 40 years. In this group, the real influence of carcinogenic factors, mainly alcohol and tobacco, is widely debated. Some authors argue that these carcinogens may also be related to the etiology of SCC in youngsters.[Citation2] Others, however, reported that many of these patients never smoked or drank alcoholic beverages, or the duration of exposure to these agents would be too short to induce malignant transformation.[Citation3]
The recent literature has given increasing attention to SCC of tongue in young adults as authors have speculated that the incidence of SCC of tongue is increasing.[Citation4,Citation5] Our understanding regarding the etiology, natural history, and optimal therapeutic management is limited due to rarity of this tumor in pediatric age group. We report a case of SCC of oral tongue in a 12-year-old boy and also review the etiological factor for SCC in pediatric age group.
Case report
A 12-year-old boy presented to the department of otorhinolaryngology for evaluation of slowly growing ulcerative lesion on his left lateral aspect of tongue (Figure ) since 8 months which was associated with pain for past 2 weeks. The patient denied any history of tobacco chewing, smoking, or alcohol intake and both his medical history and his family history were unremarkable.
Figure 1. Preoperative ulceroproliferative growth measuring about 5 × 4 cm2 at left lateral border of tongue.
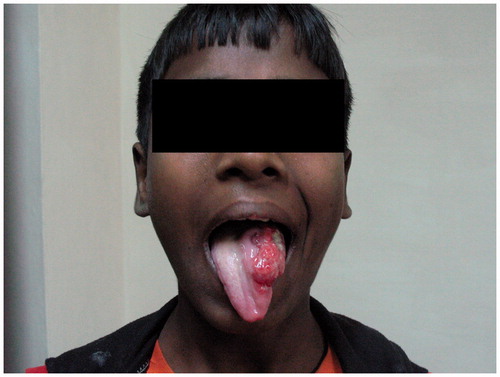
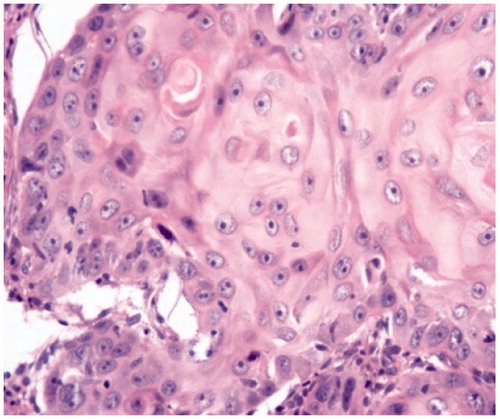
Clinical examination detected an ulceroproliferative growth of size 5 × 4 cm over left lateral aspect of tongue. The margins of ulcer were well defined and the edges were everted. The ulcer did not extend to the floor of the mouth, base of the tongue, tonsillar fossa, or retromolar trigone and did not cross the midline. On palpation, it was firm, tender, and friable with indurated base and bleeds on touch. Protrusion of the tongue was normal. Neck examination revealed a single firm, mobile lymph node of size 1.5 × 1.5 cm2 at greatest dimension at level II on left side. A wedge biopsy from the lesion was taken and sent for histopathological examination, which was reported as well differentiated squamous cell carcinoma (Figure ).
Figure 2. H&E-stained (20×) photomicrograph showing keratinization, intercellular bridge, and mild pleomorphism consistent with well-differentiated squamous cell carcinoma.
The results of all routine blood and radiological examinations were within normal limits. MRI of the face and neck revealed an ill-defined mass lesion involving the left anterolateral aspect of the tongue measuring approximately 5 × 3.5 cm2 in size. The lesion demonstrated hypo intense signal on T1, hyper intense signal on fat saturated T2-weighted images, and post-contrast imaging revealed moderate enhancement of the lesion. The mass lesion shows extension across the midline. Ipsilateral cervical lymphadenopathy noted involvement of level I, level II, and level III and the largest being of 1.7 × 1.5 cm2 at level II on left side.
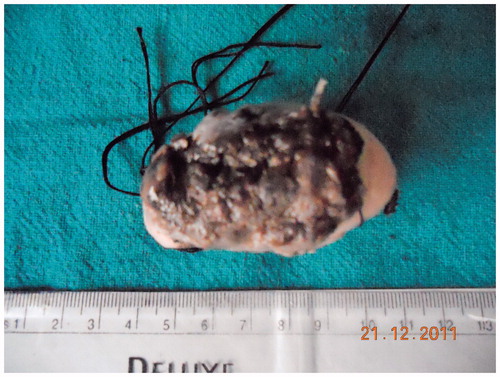
Left hemiglossectomy and modified radical neck dissection type I with primary repair of the tongue was done (Figure ). The histopathological examination report showed well-differentiated squamous cell carcinoma of the tongue with margins free from tumor. Level II lymph node was found to be involved by the disease; however; other group of lymph nodes (I, III, IV, and V) were free from disease.
After surgery, patient was sent to radiation oncology department for postoperative chemoradiation. The patient was disease free after 4 years of follow-up.
Discussion
SCC of the oral tongue is rare in young adults. Only 1–6% of patients were with SCC under the age of 40 years. The occurrence in children and adolescents is rare.[Citation3] Only scattered small series, individual case reports, and references to patients in this age group exist. Atual et al. [Citation4] in Finland found that percentage of SCC of tongue cases occurring in young adults increased from 3% per year for the decade 1953–1962 to 7% per year for the decade 1983–1992. In India, Patel et al. [Citation5] reported 5 cases of SCC of anterior tongue, the youngest of the reported case was 10-year-old male child.
The etiological factors of SCC in young patients have been widely debated. The possibility of the existence of a carcinogenic effect of tobacco and alcohol in young patients is low because in this age group the exposure time would be relatively short for establishment of a cause-effect relation.[Citation3] Some of the possible factors suggested are genetic predisposition (Fanconi’s anaemia, xeroderma pigmentosa, KID ‘keratosis, ichthyosis and deafness’ syndrome), previous viral infections, feeding habits, immunodeficiency states, occupational exposure to carcinogenic elements, socioeconomic condition, and oral hygiene and trauma. Two theories have proposed, but not proven, to explain the development of oral cancer in children: the passage of carcinogens from mother to fetus across the placenta and development from the epithelium derived from the first branchial arch.[Citation6] According to some authors, there can be three categories of etiological factors of SCC in children: (1) the immunosuppression induced by hemopathia (Fanconi’s anaemia) [Citation7] or by chemotherapy allows viral infection with HPV [Citation7]; (2) the genodermatosis diseases such as xeroderma pigmentosum [Citation8] or KID syndrome (keratosis, ichthyosis, deafness) [Citation9] may predispose to SCC; and (3) a group characterized by no particular personal or family history. Such cases are not seen frequently.
In the present report, the patient did not report any smoking or drinking habits. His medical history was not significant either. In this case, immunologic and genetic analyses were negative and there was no specific family history.
There is still controversy regarding the prognosis of SCC in young patients. Several studies have shown that young patients tend to present a greater loco-regional recurrence rate and a smaller survival rate when compared to that of older patients [Citation1,Citation3] whereas others have described a similar prognosis for both age ranges.[Citation4,Citation8]
Conclusion
This report emphasizes the fact that oral SCC can occur at young age and must be considered for suspicious lesions also in children. It further points out the need to search for other etiological factors than alcohol, smoking and tobacco consumption in the pediatric age group.
Disclosure statement
There is no conflict of interest between authors and, there is no sponsorship or source of funding.
References
- Friedlander PL, Schantz SP, Shaha AR, et al. Squamous cell carcinoma of the tongue in young patients: a matched-pair analysis. Head Neck. 1998;20:363–368.
- Oliver RJ, Dearing J, Hindle I. Oral cancer in young adults: report of three cases and review of the literature. Br Dent J. 2000;188:362–365.
- Burzynski NJ, Flynn MB, Faller NM, et al. Squamous cell carcinoma of the upper aerodigestive tract in patients 40 years of age and younger. Oral Surg Oral Med Oral Pathol. 1992;74:404–408.
- Atual S, Grenman R, Laippala P, et al. Cancer of the tongue in patients younger than 40 years. A distinct entity? Arch Otolaryngol Head Neck Surg. 1996;122:1313–1319.
- Patel DD, Dave RI. Carcinoma of the anterior tongue in adolescence. Cancer. 1976;37:917–921.
- Newman AN, Rice DH, Ossoff RH, et al. Carcinoma of the tongue in persons younger than 30 years of age. Arch Otolaryngol. 1983;109:302–304.
- Kaplan MJ, Sabio H, Wanebo HJ, et al. Squamous cell carcinoma in the immunosuppressed patient: Fanconi’s anemia. Laryngoscope. 1985;95:771–775.
- Heut-Lamy P, Dereure O, Dagrave B, et al. Carcinome lingual aucours d’un xeroderma pigmentosum. Ann Dermatol Venereol. 1992;119:980.
- Lancaster L Jr, Fournet LF. Carcinoma of the tongue in a child: report of case. J Oral Surg. 1969;27:269–270.