Abstract
Tuberculosis (TB) is one of the primary infectious diseases in the world and is accountable for more than two million deaths and nine million new cases every year. Multidrug-resistant tuberculosis (MDR-TB) is a form of tuberculosis that is resistant to two or more of the primary drugs (e.g. isoniazid and rifampin) used for the management of tuberculosis. The aminoglycoside group is the major drug group which is used in the treatment of patients with TB and can lead to hearing loss as they are ototoxic in nature. Here, we describe three patients with MDR-TB who were under medication for the same, i.e. aminoglycoside drug group along with few more antibacterial agents and anti-tuberculosis agents. The results of audiological evaluation suggested that all the patients considered for the study had the bilateral sensorineural hearing loss, where 2, 4 and 8 kHz were more affected compared to 250, 500 and 1 kHz. Other symptoms reported were tinnitus, episodic vertigo, decreased vision, general body ache, reduced appetite and insomnia.
Introduction
Tuberculosis (TB) is a disease triggered by bacteria (Mycobacterium tuberculosis) that are spread through the air from person to person. It is an infectious disease caused by droplet infection and if not treated appropriately, the disease can be lethal. It is accountable for more than two million deaths and nine million new cases every year [Citation1]. Drugs resistance is the temporary or permanent ability of an organism to remain viable or to multiply in the presence of the concentration of a drug which would normally inhibit or destroy its growth. Single drug resistance had been witnessed soon after the introduction of anti-tuberculosis agents in the treatment of tuberculosis. Drug resistance is the main cause of concern, especially poly-resistance, multidrugs resistance and extensive drug resistance which often lead to the failure of treatment with the conventional and first-line short course drugs [Citation2]. Multidrug-resistant tuberculosis (MDR-TB) is a form of tuberculosis that is resistant to two or more of the primary drugs (e.g. isoniazid and rifampin) used for the treatment of tuberculosis [Citation3]. It has been reported in the literature that the treatment of MDR-TB demands the use of second-line injectable anti-TB drugs which are ototoxic in nature [Citation4].
In India, the treatment of MDR-TB patients is carried out as per DOTS-Plus Guidelines provided by Central TB Division, Directorate General of Health Services, Ministry of Health & Family Welfare; Government of India. The drugs are given single daily dosage under directly observed treatment (DOT) and the dosages are decided according to the weight. Also, dosages are decided, depending on the presence of other medical illness or organ dysfunction for which for appropriate medical investigation will be required [Citation5].
Ototoxicity means damage to the ear due to the effect of various chemical agents, especially drugs. Ototoxic drugs affect the vestibular and cochlear part of the inner ear and it leads to hearing loss by affecting the cochlear hair cells, this may lead to hearing loss of the high frequency. Most ototoxic medication belongs to a class of the platinum-based anticancer drugs and aminoglycosides antibiotics [Citation6]. Other kinds of antibiotics can also be ototoxic, such as capreomycin, vancomycin and erythromycin. The aminoglycoside group is the major drug group which is used in the treatment of patients with TB. Previous studies have reported that aminoglycoside antibiotics (streptomycin, kanamycin, gentamicin, tobramycin, and neomycin) can lead to hearing loss. The incidence of aminoglycoside antibiotic-induced deafness (AAID) has increased over recent years and is a major cause of deafness in China [Citation3]. The World Health Organization (WHO) estimates that there are 650,000 cases globally of MDR-TB. Studies have reported MDR-TB patients often to be treated for at least 18–24 months with the second-line TB drugs to develop sensorineural hearing loss [Citation7]. Also, hearing loss pattern has been reported to be gradual and bilaterally symmetrical [Citation8]. The case reports presented here do not show the similar trend as reported in previous studies and hence, these case reports would provide us with some fresh information about the hearing loss in MDR-TB patients. Also, there is a lack of research in this particular condition in the Indian context.
Case report
Case 1 (C1)
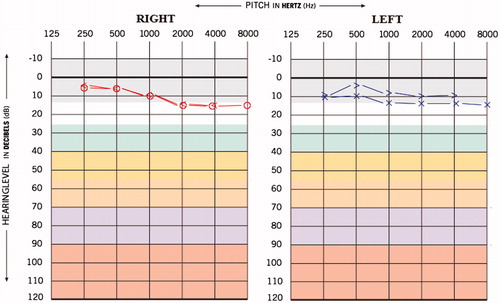
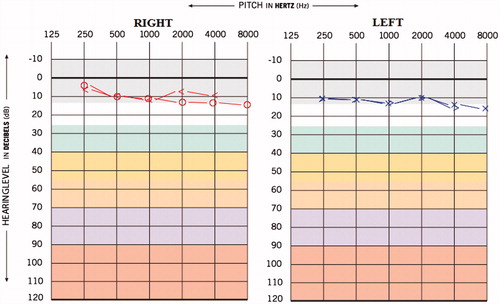
A 25-year-old male was admitted to the Department of Audiology with the complaint of reduced hearing sensitivity in both ears since 6 months, ringing sensation in the ears since 4 months and attacks of episodic vertigo for the duration of 2–3 seconds. The individual was diagnosed to have MDR-TB from last 15 months. The patient was under medication and medicines administered were Inj. Kanamycin – 750 mg IM-OD (aminoglycoside), Tab. Levofloxacin – 750 mg OD (fluoroquinolone antibiotics), Tab. Ethionamine – 1000 mg OD (antibacterial agent), Tab. Pyrazinamise – 1500 mg OD (anti-tuberculosis agent) and Tab. Ethambutol – 1200 mg OD (antibacterial agent). The individual was on these drugs for last 10 month. The patient reported hearing difficulty and other symptoms were initiated after 9 months of MDR-TB medication. The hearing screening was done prior to the administration of MDR-TB medication, and the hearing thresholds obtained for both the ears were within normal limits (Figure ). The post medication audiological evaluation was carried out at our department; the pure tone average (PTA) obtained (by using conventional three frequency average, i.e. 500 Hz, 1 kHz, and 2 kHz) was 20 and 18.3 dBHL for right and left ear, respectively, and the provisional diagnosis made was bilateral minimal steeply sloping sensorineural hearing loss (Figure ). Also, the pure tone average (PTA4) obtained (by taking the average of four frequencies, i.e. 500 Hz, 1 kHz, 2 kHz and 4 kHz) was 31.2 and 28.7 dBHL for right and left ear, respectively, which suggest bilateral mild sensorineural hearing loss. However, it can be extrapolated from the Figure , that high frequencies (i.e. 4 and 8 k) are more affected compared to other frequencies.
Case 2 (C2)
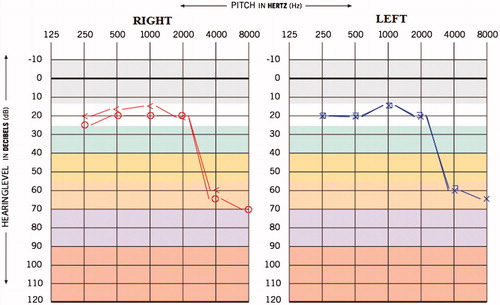
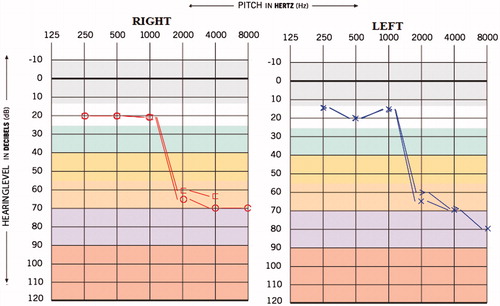
A 31-year-old male reported to our department with the complaint of reduced hearing sensitivity in the ears, ringing sensation in the ears, decreased vision, general body ache, reduced appetite since last 5 months. The individual was diagnosed to have MDR TB since last 7 months and was under medication for the same. In this particular patient, the hearing difficulty and associated symptoms began after 2 months of medication. The medications administered were Streptomycin injection 20 ml weekly (aminoglycoside), Tab. Ethinomide 500mg OD, PAS powder 10 gm H.S (anti-tuberculosis agent), Tab. Levofloxacin 250 mg OD (fluoroquinolone antibiotics), Tab. Pyrazinomide 250 mg OD, Tab. Echambuto 800 mg OD and Tab. Pyridoxin 100 mg OD. The result of hearing of screening done before the initiation of medication was the bilateral hearing sensitivity within normal limits (Figure ). The post medication pure tone average (PTA) obtained was 43.3 and 30 dBHL for right and left ear, respectively, and the provisional diagnosis made was ‘Moderate sensorineural sloping hearing loss’ in right ear and ‘Mild sensorineural sloping hearing loss’ in left ear (Figure ). The pure tone average (PTA4) obtained was 60 and 42.5 dBHL for right and left ear, respectively, which suggest ‘Moderately severe sensorineural hearing loss’ in right ear and ‘Moderate sensorineural hearing loss’ in left ear
Case 3 (C3)
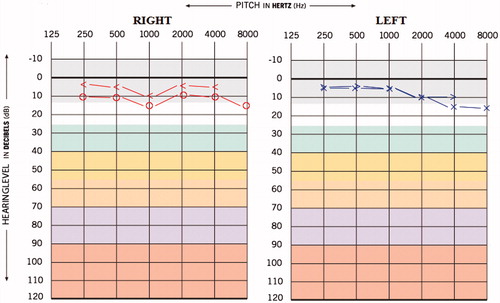
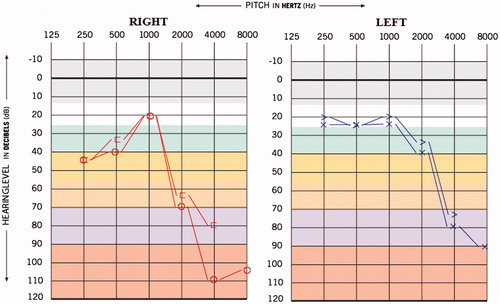
This 27-year-old female reported to us with the complaint of reduced hearing sensitivity in ears, insomnia, reduced social interaction and tinnitus in both the ears since 3 years. Also, the patient had the history of suicide attempt and all these symptoms arose after the patient started with TB medication. The patient was diagnosed with MDR-TB four years back and medication administered were Inj. Kanamycin 500 mg IM-OD, Tab. Moxiflox 400 mg OD, Cap. Clofazimine 200 mg OD, Ethionomide 500 mg OD (antibacterial agent), Ethambutol 800 mg OD (antibacterial agent), Cap.Cycloserine 500mg OD (antibacterial agent) and Levoflexacin 750 mg OD (fluoroquinolone antibiotics). The hearing and other psychiatric problems were reported after one year of MDR-TB medication. The results of hearing screening carried out prior to initiation of MDR-TB medication was the bilateral hearing sensitivity within normal limits (Figure ). The pure tone average obtained was 35 and 33.3 dBHL for right and left ears, respectively, and the provisional diagnosis made was ‘bilateral mild sloping sensorineural hearing loss’ (Figure ). The pure tone average (PTA4) obtained was 43.7 and 42.5 dBHL for right and left ears, respectively, which suggest bilateral moderate hearing loss.
Discussion
The patients who do not take their TB medicine regularly; or do not take all of their TB medicine as advised and/or developed TB again, after having taken TB medicine in the past are at risk of having MDR-TB. In the present study, we have reported three patients with MDR-TB and all of them were using aminoglycoside group of drug, i.e. in C1, the aminoglycoside used was Inj. Kanamycin 750 mg IM-OD; in C2 streptomycin injection 20 ml weekly and in C3 Inj. Kanamycin 500 mg IM-OD was used. Along with aminoglycoside, other drug groups used were fluoroquinolones (levofloxacin, moxifloxacin), old bacteriostatic second line anti-tuberculosis agents (ethionamide, cycloserine) and anti-tuberculosis agent (Clofazimine, clarithromycin).
The participants considered for the study had no history of any otological symptoms, audiological and neurological impairment; also, none of them had the history of noise exposure when the TB medication was initiated. To rule out the presence of any otological and audiological impairments, pure tone audiometry and tympanometry were done and results obtained were the bilateral hearing threshold with normal limits and A-type tympanogram, respectively, for all the above-mentioned cases. All ethical standards were met for participant selection and their participation. Prior to testing, a written consent was obtained from the participants after explaining the purpose of the study. The audiometer used in the present study was 2-channel AC40 of Interacoustics (Middelfart, Denmark) and tympanometry used was MI44 of MAICO (Salzufer, Berlin, Germany). Also, the pre- and post-medication recordings were done at the same setup using same instrumentation.
We have observed that all of our patients have reported reduced hearing sensitivity in both ears followed by the administration of MDR-TB drugs, where aminoglycoside group is ototoxic in nature; along with reduced hearing sensitivity, other symptoms such as tinnitus, episodic vertigo, decreased vision, general body ache, reduced appetite, and insomnia were also reported. Likewise, Saunders et al. [Citation9] have reported that the ototoxic and nephrotoxic effects of aminoglycosides became evident shortly after their introduction and have limited their use although in developing countries they are still widely used due to their broad antimicrobial spectrum and low cost. Although, similar results were not seen in our subjects; in C1 the symptoms were initiated after 2 months; in C2 after 9 months and in C3 after 1 year of medication. Therefore, the onset of hearing loss and other symptoms can vary from patient to patient.
Another major finding of the present study would be the presence of high-frequency sensorineural hearing loss in each of three patients irrespective of the duration of the drug intake. Duggal and Sarkar [Citation7] have suggested that MDR-TB patients often have to be treated for at least 18–24 months with the 2 line TB drugs; which leads the patients to be at high risk of developing aminoglycoside-induced sensorineural hearing loss. Although the duration of drug intake might not directly be related to the degree of hearing loss, C1 was under medication for 4 months and had mild degree of HL; C2 was under medication for 7 months and had mild-moderate degree of HL; while C3 was under medication for 4 years and had only mild degree of HL. Hence, there is a possibility that shorter duration of aminoglycoside intake can also lead to hearing loss.
O’Donnell et al. [Citation8] have reported the presence of bilateral symmetrical hearing loss in these patients; in present study C1 and C3 have shown the bilateral symmetrical hearing loss while C2 has the asymmetrical degree of hearing loss. Vaamonde et al. [Citation10] have reported that aminoglycosides target the sensorineural epithelium of the inner ear. Outer cochlear hair cells are more vulnerable to injury than inner hair cells; the basal region of the cochlea is more prone than the apical region and loss of cochlear hair cells causes secondary degeneration of the auditory nerve [Citation11]. This can be clinically expressed as the high-frequency hearing loss; with increased exposure, this progress to involve the lower frequencies. Our finding also suggests the presence high-frequency loss in all the patients, where 2, 4 and 8 kHz were majorly affected compared to 250, 500 and 1 kHz. As the progression of the destruction of outer hair cell spread from high- to low-frequency regions, it goes undetected till conventional audiometric testing frequency start deteriorating. For early detection of hearing loss two audiological methods can be used: firstly high-frequency audiometry (0.5–20 Khz). and secondly OAE. Each present some merits and demerits; however, OAE overshadow high-frequency audiometry with these extra reasons, starting with less time consuming, OAE is a noninvasive measure of cochlear outer hair cell, more sensitive measure for the early detection of hearing loss than conventional PTA, secondly no active participation of subjects, can be easily performed in younger population and more importantly provide frequency-specific information. Furthermore, Reavis et al. [Citation12] have suggested that the DPOAE hit rate was 78% in ear with hearing loss confirmed by high-frequency audiometry (0.5–20 kHz); likewise few more authors have also reported that OAE are more sensitive monitoring tool for detecting drug-induced ototoxicity [Citation13]. For the above-mentioned reasons, it can be strongly recommended that OAE must be a criterion in the protocol of evaluation and monitoring of TB patients.
Conclusion
To conclude, MDR-TB medication induces hearing loss in these patients; although the onset of hearing loss; type and degree of hearing loss and other signs and symptoms varies from individual to individual. Hence, to attain precise information about this condition, a detailed investigation should be done by controlling different variables such as the age of the patient; onset of TB and/or MDR-TB; effect different medications and their cocktails on a larger population which will further help the professionals in the better intervention of the patients with MDR-TB. Also, otoacoustic emission (OAE) must be added to the protocol of the early identification of hearing loss in these patients as OAE is a more sensitive tool compared to pure tone audiometry (PTA) to detect outer hair cell damage which is prominently damaged by aminoglycoside drug group. Further, a study can be done to see neural integration in these patients using electrophysiological tests.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Harries AD, Dye C. Tuberculosis. Ann Trop Med Parasitol. 2006;100:415–431.
- Calver AD, Murray M, Strauss OJ, et al. Emergence of increased resistance and extensively drug-resistant tuberculosis despite treatment adherence South Africa. Emerg Infect Dis. 2010;16:264.
- World Health Organization. WHO media centre [Internet]. 2013. Available from: http://www.who.int/mediacentre/factsheets/fs300/en/
- Statistics South Africa. Statistical release P0302 [Internet]. [cited 2016 Nov 15] Available from: http://www.statssa.gov.za/publications/P0302/P03022011.pdf
- DOTS-Plus Guidelines. Revised National Tuberculosis Control Program (RNTCP). Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare; [Internet]. 2010. Available from: http://health.bih.nic.in/Docs/Guidelines/Guidelines-DOTS-Plus.pdf
- Schacht J, Talaska AE, Rybak LP. 2012. Cisplatin and Aminoglycoside Antibiotics: Hearing Loss and Its Prevention. Anat Rec (Hoboken). 295:1837–1850.
- Duggal P, Sarkar M. Audiologic monitoring of multidrug resistant tuberculosis patients on aminoglycoside treatment with long term follow up. BMC Ear Nose Throat Disord. 2007;7:5.
- O’Donnell EP, Scarsi KK, Scheetz MH, et al. Risk factors for aminoglycoside ototoxicity in adult cystic fibrosis patients. Int J Antimicrob Agents. 2010;36:94–95.
- Saunders JE, Greinwald JH, Vaz S, et al. 2009. Aminoglycoside ototoxicity in Nicaraguan children: patient risk factors and mitochondrial DNA results. Otolaryngol Head Neck Surg. 140:103–107.
- Vaamonde P, Castro C, García-Soto N, et al. Tuberculous otitis media: a significant diagnostic challenge. Otolaryngol Head Neck Surg. 2004;130:759–766.
- Selimoglu E. Aminoglycoside-induced ototoxicity. Curr Pharm Des. 2007;13:119–126.
- Reavis KM, Phillips DS, Fausti SA, et al. Factors affecting sensitivity of distortion-product otoacoustic emissions to ototoxic hearing loss. Ear Hear. 2008;29:875–893.
- Stavroulaki P, Apostolopoulos N, Segas J, et al. Evoked otoacoustic emissions–an approach for monitoring cisplatin induced ototoxicity in children. Int J Pediatr Otorhinolaryngol. 2001;59:47–57.