Abstract
Myofibroma of the mandible is a rare, benign neoplasm with current treatment consisting of surgical excision. Various approaches have been reported from enucleation or curettage to segmental mandibulectomy and free tissue transfer techniques. There are no reports in the literature of observation for spontaneous regression. An 11-month-old female was diagnosed with mandibular myofibroma. During workup of the mass, interval imaging demonstrated a decrease in size of the lesion. To avoid an extensive surgical resection, observation and repeat imaging was recommended to the patient’s family. Given ongoing decrease in the size of the lesion and its asymptomatic nature, the patient has continued to be observed clinically and radiographically. This is the first report of mandibular myofibroma managed with observation wherein there seems to have been spontaneous regression of the mass without any intervention.
Introduction
Infantile myofibromatosis is a rare, benign neoplasm of contractile myoid cells which can arise from soft tissue, bone, or visceral organs. It can present either as a solitary or a multicentric mass, with the former comprising approximately 50% of cases [Citation1]. Solitary myofibromas generally present as a cutaneous or subcutaneous nodule. These nodules are uncommon in the maxillofacial region. However, when present, they tend to show a penchant for the mandible [Citation2]. Rarely, intraosseous myofibromas of the mandible have been identified with nearly 40 cases described in the literature [Citation3]. The treatment of choice for solitary tumours typically involves surgical excision [Citation4]. While spontaneous regression of soft tissue myofibromas has been described, to the best of our knowledge, there are no reports of spontaneous regression of a mandibular myofibroma. We present a case of a solitary intra-osseous myofibroma of the mandible in a child wherein there appears to be spontaneous regression.
Case presentation
Patient information
The patient, an 11-month-old female, presented to an outpatient Pediatric Otolaryngology clinic for urgent assessment of a left mandible mass incidentally identified on magnetic resonance imaging (MRI) for workup of a forehead subcutaneous lesion which was ultimately diagnosed as an infantile hemangioma. The patient had not had any prior difficulty with feeding, respiration, growth or sleep. She had no other prior medical history and was developmentally normal. There was no family history of congenital masses or syndromes.
Clinical findings
On examination, the patient had no evidence of symptoms from this lesion. There was no trismus present. There was subtle fullness to the left cheek and on intra-oral palpation some bulging to the left retromolar trigone. There were no abnormalities of the overlying mucosa.
Diagnostic assessment
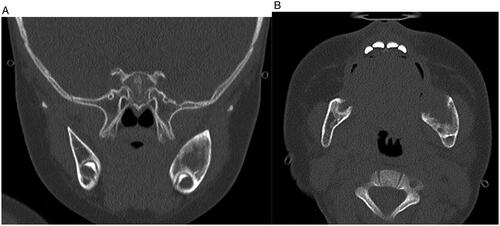
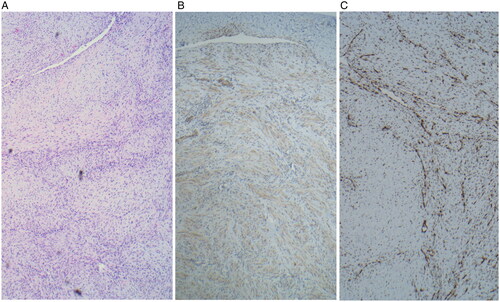
MR imaging revealed a 3.2 cm anterior-posterior (AP) × 2.3 cm craniocaudad (CC) × 1.8 cm transverse (T) mass arising from the left mandibular ramus with well-defined borders. There was involvement of the angle and body of the mandible but no involvement of the condylar or coronoid processes. It demonstrated low T1, intermediate T2 signal intensity and contrast uptake. There was displacement of the medial pterygoids medially and the masseter laterally (Figure ) The patient had also undergone whole-body MRI which did not demonstrate any additional lesions or lymphadenopathy in the neck, chest, abdomen, or pelvis. To obtain a definitive diagnosis, intra-operative specimens were obtained trans-orally using two 7 mm punch biopsies in the region of the retromolar trigone. While intraoperative pathology consultation suggested spindle cell lesion, the final diagnosis was mandibular myofibroma as microscopic analysis revealed well circumscribed, nodular neoplasms characterized by a distinctive biphasic growth pattern of immature appearing, plump, spindled tumor cells associated with hemangiopericytoma-like branching blood vessels. There was a clear architectural pattern of nodules and fascicles of variably hyalinized, myoid appearing cells. Mitotic figures were low and by immunohistochemistry the tumor cells were positive for smooth actin marker H-caldesmon, negative for desmin. ERG and CD34 highlighted the vascular spaces. The tumor cells were negative for S100, CKAe, EMA, HMB45 and Sox10 (Figure ). CT imaging was then undertaken for surgical planning. CT imaging better characterized the bony involvement to be lytic in nature with greater bony destruction at the medial aspect of the lesion without violation of the cortex. There was slight decrease in size as measured on the CT compared to the MRI (2.0 cm AP × 2.1 cm CC × 1.5 cm T) (Figure ) Given this decrease in size, surveillance was recommended with follow-up MR imaging to be conducted at 6, 12 and 24 months following the initial study.
Figure 1. (A and B) MRI in the coronal and axial view seen in T2 demonstrating a 3.2 cm anterior-posterior (AP) × 2.3 cm craniocaudad (CC) × 1.8 cm transverse (T) mass arising from the left mandibular ramus with well-defined borders and with involvement of the angle and body of the mandible without involvement of the condylar or coronoid processes.
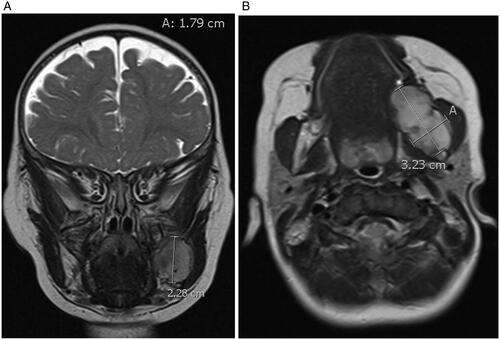
Figure 2. Microscopy specimens shows (A) a well circumscribed nodular neoplasms at 40× characterized by a distinctive biphasic growth pattern of immature appearing, plump, spindled tumor cells associated with hemangiopericytoma-like branching blood vessels. There is a clear architectural pattern of nodules and fascicles of variably hyalinized, myoid appearing cells. Mitotic figures are low. (B) Immunohistochemistry reveals tumor cells positive for smooth actin marker H-caldesmon and (C) negative for desmin.
Follow-up and outcomes
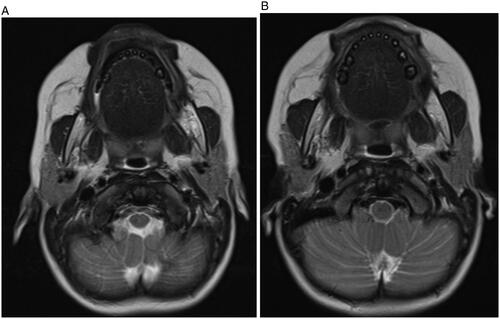
Repeat MR imaging at 17, 23 and 34 months of age revealed further decreases in mass size along with decreased signal intensity, enhancement and bony expansion (Figure ). As there has been progressive decrease in size without any evidence of upper aerodigestive tract symptoms or impact on the child’s growth and development, she is undergoing ongoing clinical observation with MR imaging surveillance. At the patient’s more recent clinical and radiographic evaluations, molar growth in the region of the mass was noted symmetric to the contralateral side. The patient remains asymptomatic.
Figure 4. (A and B) Axial views of MR imaging in T2 window at 12 months (A) and 24 months (B) after diagnosis revealed further decrease in mass size along with decreased signal intensity, enhancement and bony expansion. There is only mild residual expansion of the left angle of mandible after 24 months.
Discussion
Solitary myofibromas are a rare spindle-cell lesion uncommonly found in the head and neck. The mandible is the most common location within the oral cavity [Citation5]. Intraosseous presentations are rare, but can typically occur in the posterior mandible or calvarium [Citation6,Citation7]. Less than 40 documented cases of mandibular myofibromas are reported in the literature [Citation3].
Many cases of intraosseous myofibromas tend to present as an asymptomatic enlarging mass discovered incidentally upon assessment for unrelated health concerns, as seen in our patient who underwent imaging for an unrelated mass. Should these myofibromas incite symptoms, they usually present as painless swelling, facial asymmetry, trismus, mental paresthesia or dental complications such as expansion and resorption of bone plates, tooth dislocations or growth abnormalities [Citation6].
On CT imaging, they tend to show unilocular radiolucency with well demarcated borders and bony expansion; however, they may also show a multilocular appearance [Citation3]. MR imaging demonstrates hypointensity on T1 sequences, mixed intensity on T2 imaging, and strong contrast uptake [Citation8]. Our case demonstrates similar findings on CT and MRI.
On histopathology, myofibromas reveal an alternating pattern of proliferating spindle or ovoid-shaped myofibroblasts with minimal cytoplasm surrounded by an extracellular matrix with areas of smaller and densely packed spindle or round-shaped myofibroblasts with eosinophilic cytoplasm. Immunohistochemistry (IHC) is positive for vimentin and αSMA and negative for desmin, S100, and CD68 [Citation3]. On intraoperative pathology, our case was reported to be a spindle cell lesion. After complete histopathologic evaluation including immunohistochemistry, the lesion was diagnosed as myofibroma.
These benign lesions are traditionally treated by conservative surgical excision. In a literature review by Berthold et al. 20 patients across 17 reports of mandibular myofibroma were all treated with some form of surgical excision, ranging from enucleation or curettage to composite resection [Citation9,Citation10]. Similarly, retrospective chart review by Abramowicz et al. included seven cases of intraosseous myofibromas of the mandible, all treated with enucleation and curettage [Citation11]. Later case reports continue to report treatment with surgical excision. Only a solitary infantile intraosseous myofibroma of the zygomatico-maxillo-orbital complex has been reported to regress spontaneously without any surgical intervention [Citation12].
The rate for myofibroma recurrence is reported to range between 0% to 12.5% [Citation13]. Recurrences are often attributed to incomplete removal. While our patient is yet to show any evidence of progression of disease, it is unclear what the rate of recurrence is in the case of spontaneously regressing myofibromas. Ongoing follow-up is warranted.
In summary, solitary myofibroma of the mandible is a rare tumour and can present in infancy. While the majority of available case reports on this entity report conservative surgical excision as the standard of care, we present a case wherein there appears to be spontaneous regression of the mass without any intervention. To the best of our knowledge, this is the first report of mandibular myofibroma being managed with active surveillance as opposed to surgical resection. Further research is needed in this area with larger case series to clarify the role of active surveillance and specific indications for surgical intervention.
Informed consent
Written informed consent to publish this case report has been obtained from the patient’s legal guardian.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Mashiah J, Hadj-Rabia S, Dompmartin A, et al. Infantile myofibromatosis: a series of 28 cases. J Am Acad Dermatol. 2014;71(2):264–270. doi: 10.1016/j.jaad.2014.03.035.
- Lee YM, Son SM, Kim KW, et al. Solitary myofibroma of the adult mandible: a case report and review of literature. Korean J Pathol. 2014;48(4):307–310. doi: 10.4132/KoreanJPathol.2014.48.4.307.
- Sundaravel S, Anuthama K, Prasad H, et al. Intraosseous myofibroma of mandible: a rarity of jaws: with clinical, radiological, histopathological and immunohistochemical features. J Oral Maxillofac Pathol. 2013;17(1):121–125. doi: 10.4103/0973-029X.110703.
- Dhupar A, Carvalho K, Sawant P, et al. Solitary intra-osseous myofibroma of the jaw: a case report and review of literature. Children (Basel). 2017;4(10):91. doi: 10.3390/children4100091.
- Foss RD, Ellis GL. Myofibromas and myofibromatosis of the oral region: a clinicopathologic analysis of 79 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(1):57–65. doi: 10.1067/moe.2000.102569.
- Nirvikalpa N, Narayanan V. Intraosseous infantile myofibroma of the mandible. Ann Maxillofac Surg. 2011;1(1):87–90. doi: 10.4103/2231-0746.83151.
- Demir M, Yapicier O, Celik O, et al. Isolated infantile myofibroma of the calvarium: report of a case with a literature review. Childs Nerv Syst. 2024. doi: 10.1007/s00381-024-06289-9.
- Newaz ZA, Pannu V, Kennedy J, et al. Imaging features of intraosseous myofibroma of the jaws: a case report and literature review. J Adv Radiol Med Image. 2016;1(1):102.
- Berthold RCB, Zanella TA, Casagrande LCO, et al. Intraosseous myofibroma of the jaw: review of the literature. Stomatos. 2015;21(40):27–35.
- Maby A, Guay B, Thuot F. Infantile myofibromatosis treated by mandibulectomy and staged reconstruction with submental flap and free fibula flap: a case report. J Otolaryngol Head Neck Surg. 2019;48(1):14. doi: 10.1186/s40463-019-0333-z.
- Abramowicz S, Simon LE, Kozakewich HP, et al. Myofibromas of the jaws in children. J Oral Maxillofac Surg. 2012;70(8):1880–1884. doi: 10.1016/j.joms.2011.09.029.
- Arab K, Maltese G, Kölby L, et al. Infantile myofibroma of the zygomatico-maxillo-orbital complex: case report with spontaneous regression. Oral Maxillofac Surg Cases. 2016;2(4):35–39. doi: 10.1016/j.omsc.2016.11.001.
- Haspel AC, Coviello VF, Stevens M, et al. Myofibroma of the mandible in an infant: case report, review of the literature, and discussion. J Oral Maxillofac Surg. 2012;70(7):1599–1604. doi: 10.1016/j.joms.2011.07.006.