Abstract
Although the Gelechioidea is the second most species-rich group of Lepidoptera, comparatively few mitochondrial genomes (mitogenomes) have been sequenced in this superfamily. Here, we determined the complete mitogenomic sequence of the gelechioid Hieromantis kurokoi (Lepidoptera: Stathmopodidae) as the basis for a future study of the phylogeny of butterflies and moths (clade Ditrysia). The H. kurokoi genome was 15,208 bp with a typical set of genes (13 protein-coding genes [PCGs], two rRNA genes and 22 tRNA genes) and one major non-coding A + T-rich region. The cytochrome c oxidase subunit 1 (COI) gene had a CGA start codon, which is the start codon for this gene in the majority of lepidopteran species, whereas other PCGs began with an ATN codon. A 360 bp-long A + T-rich region harbored the blocks of conserved sequences that are typically found in lepidopteran insects. Phylogenetic analysis using the 13 PCGs and Bayesian inference (BI) and maximum-likelihood (ML) methods indicated that H. kurokoi belonged to the family Stathmopodidae and grouped together with the within-familial species Atrijuglans hetaohei with the highest nodal support (BI, 1.0; ML, 100%).
The superfamily Gelechioidea is the second most species-rich group of Lepidoptera and is important for the understanding of the higher phylogeny of the Ditrysia clade (Kaila et al. Citation2011; van Nieukerken et al. Citation2011). Nevertheless, prior to this study, only seven mitochondrial genomes (mitogenomes), representing six of the 19 families, have been sequenced (Park et al. Citation2016; Timmermans et al. Citation2014; Zhao et al. Citation2016). Thus, more species from a diverse taxonomic group will be essential for mitogenome-based phylogenetic studies.
In this study, we sequenced the mitogenome of the gelechioid Hieromantis kurokoi (Lepidoptera: Stathmopodidae) (Sohn Citation2007). One adult was captured at Joongge-ri in Jeollabuk-do Province, South Korea (35°39′14.9′′ N, 126°32′14.9′′ E). A voucher specimen was deposited in Chonnam National University, Gwangju, Korea. Total DNA was used as the template to amplify three long overlapping fragments (COI-ND4, ND5-lrRNA and lrRNA-COI). Subsequently, 26 short overlapping fragments were amplified using the long fragments as templates. All primer used were Lepidoptera-specific primers designed previously (Kim et al. Citation2012).
The complete mitogenome of H. kurokoi (GenBank accession number KU605775) was 15,208 bp and consisted of two rRNAs, 22 tRNAs, 13 protein-coding genes (PCGs) and one major non-coding region (referred to as the A + T-rich region). The gene arrangement of H. kurokoi is identical to that of other ditrysian Lepidoptera, including gelechioids, that have the order trnM-trnI-trnQ (where the underline indicates a gene inversion) between the A + T-rich region and ND2 (Kim et al. Citation2011; Park et al. Citation2016; Timmermans et al. Citation2014; Zhao et al. Citation2016), instead of the ancestral trnI-trnQ-trnM order found in the majority of insects (Boore Citation1999).
Twelve of the 13 PCGs started with ATN codons (data not shown), but the COI gene began with CGA (arginine). Ten of the 13 PCGs had a complete stop codon, but COI, COII and ND4 had T as the stop codon (data not shown). The 360 bp A + T-rich region displayed several Lepidoptera-specific features, such as the ATAGA motif and adjacent poly-T stretch, microsatellite-A/T repeat, and a poly-A stretch (data not shown).
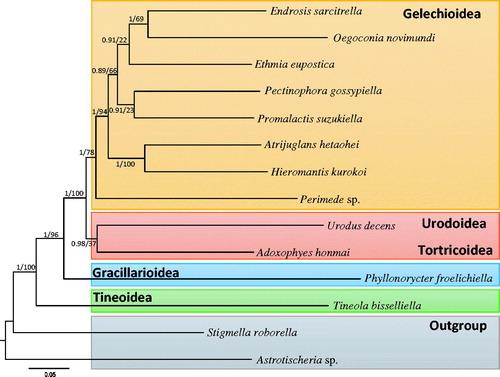
Phylogenetic analysis using nucleotide sequences of the 13 PCGs was performed with eight species of gelechioids from six families including H. kurokoi and one species from each of the Urodoidea, Tortricoidea, Tineoidea and Gracillarioidea, which are phylogenetically close to the Gelechioidea. Both Bayesian inference (BI) and maximum-likelihood (ML) methods were performed using the GTR + GAMMA + I model in CIPRES Portal v. 3.1 (Miller et al. Citation2010). The two approaches generated an identical topology, but nodal support varied (). Hieromantis kurokoi, which belongs to Stathmopodidae grouped together with the within-familial species Atrijuglans hetaohei with the highest nodal support (ML, 100%; BI, 1.0). However, Endrosis sarcitrella and Promalactis suzukiella that belong to Oecophoridae did not form the monophyletic group. Instead, E. sarcitrella formed a group with the sole member of Autostichidae, Oegoconia novimundi and P. suzukiella formed a group with the sole member of Gelechiidae, Pectinophora gossypiella, although these groups were very weakly supported by the ML method. These results may indicate that it is too early to determine within-superfamilial inferences for Gelechioidea with the currently available mitogenome data.
Figure 1. Phylogenetic tree for apoditrysian and ditrysian superfamilies, including Gelechioidea in Lepidoptera. Tree was constructed using nucleotide sequences of 13 protein-coding genes via the Bayesian inference method. The numbers at each node specify Bayesian posterior probabilities percentages by Bayesian inference method (first value) and bootstrap percentages of 1,000 pseudoreplicates by maximum-likelihood method (second value). The scale bar indicates the number of substitutions per site. One species each of Tischerioidea (Astrotischeria sp.) and Nepticuloidea (Stigmella roborella) were included as outgroups. GenBank accession numbers are as follows: Urodus decens, KJ508062 (Timmermans et al. Citation2014); Adoxophyes honmai, DQ073916 (Lee et al. Citation2006); Ethmia eupostica, KJ508047 (Timmermans et al. Citation2014); Perimede sp., KJ508041 (Timmermans et al. Citation2014); Endrosis sarcitrella, KJ508037 (Timmermans et al. Citation2014); Promalactis suzukiella, KM875542 (Park et al. Citation2014); Oegoconia novimundi, KJ508036 (Timmermans et al. Citation2014); Atrijuglans hetaohei, KT581634 (Unpublished); Pectinophora gossypiella, KM225795 (Zhao et al. Citation2016); Tineola bisselliella, KJ508045 (Timmermans et al. Citation2014); Phyllonorycter froelichiella, KJ508048 (Timmermans et al. Citation2014); Astrotischeria sp., KJ508056 (Timmermans et al. Citation2014); and Stigmella roborella, KJ508054 (Timmermans et al. Citation2014).
Funding information
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2015R1D1A3A03018119).
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Boore JL. 1999. Animal mitochondrial genomes. Nucleic Acids Res. 27:1767–1780.
- Kaila L, Mutanen M, Nyman T. 2011. Phylogeny of the mega-diverse Gelechioidea (Lepidoptera): adaptations and determinants of success. Mol Phylogenet Evol. 61:801–809.
- Kim MJ, Kang AR, Jeong HC, Kim K-G, Kim I. 2011. Reconstructing intraordinal relationships in Lepidoptera using mitochondrial genome data with the description of two newly sequenced lycaenids, Spindasis takanonis and Protantigius superans (Lepidoptera: Lycaenidae). Mol Phylogenet Evol. 61:436–445.
- Kim JS, Park JS, Kim MJ, Kang PD, Kim SG, Jin BR, Han YS, Kim I. 2012. Complete nucleotide sequence and organization of the mitochondrial genome of eri-silkworm, Samia Cynthia ricini (Lepidoptera: Saturniidae). J Asia Pac Entomol. 15:162–173.
- Lee ES, Shin KS, Kim MS, Park H, Cho S, Kim CB. 2006. The mitochondrial genome of the smaller tea tortrix Adoxophyes honmai (Lepidoptera: Tortricidae). Gene. 373:52–57.
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the gateway computing environments (GCE) workshop, New Orleans. p. 1–8.
- Park JS, Kim SS, Kim KY, Kim I. 2016. Complete mitochondrial genome of Suzuki’s Promolactis moth Promalactis suzukiella (Lepidoptera: Oecophoridae). Mitochondrial DNA. 27:2093–2094.
- Sohn, JC. 2007. Faunistic contribution to the Korean Microlepidoptera and pyralids (1)-40 species new to Korea. Tinea. 20:12–27.
- Timmermans MJ, Lees DC, Simonsen TJ. 2014. Towards a mitogenomic phylogeny of Lepidoptera. Mol Phylogenet Evol. 79:169–178.
- van Nieukerken EJ, Kaila L, Kitching IJ, Kristensen NP, Lees DC, Minet J, Mitter C, Mutanen M, Regier JC, Simonsen TJ, et al. 2011. Order Lepidoptera Linnaeus, 1758. Zootaxa. 3148:212–221.
- Zhao J, Sun Y, Xiao L, Tan Y, Dai H, Bai L. 2016. Complete mitochondrial genome of the pink bollworm Pectinophora gossypiella (Lepidoptera: Gelechiidae). Mitochondrial DNA. 27:1575–1576.
