Abstract
The complete mitochondrial genome of the white perch Morone americana is described in this study. The total length of M. americana mitogenome is 17,966 bp, consisting of 13 protein-coding genes, 22 transfer RNAs, two ribosomal RNA genes and a noncoding control region. As with several other species in Moronidae, the ND6 gene in M. americana is found within the control region rather than at the canonical position between the ND5 and CYTB genes. Phylogenetic analysis based on CYTB gene places M. americana within Moronidae and confirms its close relationship with yellow perch (M. mississippiensis). This study provides a basic resource for further research on genetic structure and demographic history of M. americana.
The white perch Morone americana is an abundant semianadromous species found along the east coast of United States (Funderburk Citation1991). They are typically found in coastal bays and estuaries, as well as freshwater and brackish lakes and ponds (Woolcott Citation1962). Because of its abundance and euryhaline distribution in western Atlantic waters, M. americana is often used as an indicator species for contaminants (King et al. Citation2004; Hiramatsu et al. Citation2006; Barnthouse et al. Citation2009). To better understand spatial and ontogenetic patterns of contaminant accumulation in this species, genetic markers are needed to elucidate fine-scale population structure and demography. However, available molecular markers for M. americana are very limited at present. Therefore, we sequenced the complete mitochondrial genome of M. americana, to provide a valuable tool for continued investigation into population structure in this species.
Our sample was collected by bottom trawl in the upper Delaware Bay, USA in 2009. Total genomic DNA was extracted using a Qiagen DNA Isolation Kit (QIAGEN Inc., Valencia, CA). In order to sequence the mitochondrial genome, we first used several pairs of custom primers to amplify mitochondrial fragments containing ATP8, ATP6, ND2, ND4L and ND5. Then, we employed a primer-walking sequencing strategy to sequence the whole mitogenome. Polymerase chain reaction (PCR) primers and protocols used for this study are available upon request. Sequencher v.4.1.1 (Gene Codes Corp., Ann Arbor, MI) was used to make the assembly. Sequence chromatograms were examined in order to detect and correct sequencing errors. Three online programs – MitoAnnotator (Iwasaki et al. Citation2013), MITOS (Bernt et al. Citation2013) and DOGMA (Wyman et al. Citation2004) – were used to provide mitogenome annotations, As some annotations showed inconsistency, manual confirmation was performed by aligning the conflicting annotations to the mitogenome of three related species: Morone saxatilis, Dicentrarchus labrax and Dicentrarchus punctatus (GenBank accession number: HM447585, KJ168065, KJ168066).
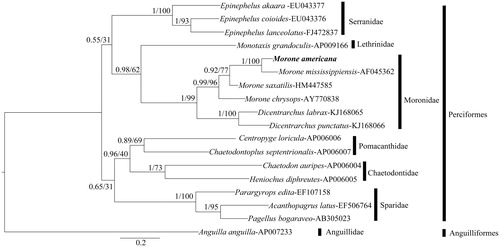
In order to confirm the validity of the draft mitogenome, the complete CYTB nucleotide sequence of white perch and 17 other teleost species were used to construct a phylogenetic tree. A maximum-likelihood (ML) tree and a Bayesian tree were constructed. The ML tree was obtained using the online program PhyML 3.0 (Guindon et al. Citation2010) with 100 bootstrap replicates. MrBayes 3.2.5 (Ronquist et al. Citation2012) was used to build the Bayesian tree. The evolutionary model in MrBayes was set to the GTR substitution model with gamma-distributed rate variation across sites and a proportion of invariant sites. In order to clarify phylogenetic relationships within the genus Morone, four species (M. americana, M. saxatilis, M. mississippiensis and M. chrysops) were included to build another tree. DNA sequences from three mitochondrial genes (partial COXI, CYTB, ND6) of each species were concatenated and used to construct ML and Bayesian trees by as described previously.
The complete mitogenome sequence of M. americana contains 17,966 bp and has been deposited in GenBank with accession number KU641485. It consists of 13 protein-coding genes, 22 transfer RNAs, two ribosomal RNAs genes and a noncoding control region. Gene order is identical to the congeneric species M. saxatilis, except that annotated genome for the latter (HM447585) contains an additional tRNA-Ser. This inferred tRNA was not detected by MitoAnnotator, MITOS or tRNAScan-SE (Schattner et al. Citation2005), did not form a conventional tRNA-folding structure using RNAFold (Gruber et al. Citation2008) and had no match in the M. americana mitogenome. Therefore, it is unlikely to represent a real tRNA.
As with several other species in Moronidae (Williams et al. Citation2012; Tine et al. Citation2014), the ND6 gene in M. americana was found within the control region rather than the canonical position between the ND5 and CYTB genes. The other genes were found have a typical vertebrate mitochondrial gene arrangement. The whole base composition of M. americana was 29.1% C, 27.8% A, 26.9% T and 16.2% G. All the protein-coding genes began with ATG as the start codon, except for COXI, which started with GTG. For the stop codon, five genes used TAA, while the other eight genes had incomplete stop codons (TA or T), which were presumably completed as TAA by post-transcriptional polyadenylation (Anderson et al. Citation1981). Most of the genes were encoded on the H-strand, except for eight tRNA genes and ND6. The origin of L-strand replication (OL) in M. americana was found located between tRNAAsn and tRNACys genes within a cluster of five tRNA genes (tRNA-Trp, tRNA-Ala, tRNA-Asn, tRNA-Cys and tRNA-Tyr). Although the control region was separated by ND6 into two parts, we still identified one termination-associated sequence (TAS), one central conserved sequence block (CSB-D) and three conserved sequence blocks (CSB-1, CSB-2, CSB-3). We also found a 121bp tandem repeat sequence with eight complete repeats and one truncated repeat. In another M. americana control region sequence (HM447588) only four complete repeats and one truncated repeat were observed. Size heteroplasmy in the control region has also been documented in M. saxatilis (Chapman Citation1990; Wirgin et al. Citation1995; Williams et al. Citation2012) and D. labrax (Cesaroni et al. Citation1997).
The ML tree and Bayesian inference trees had the same topology (), with the Perciformes and Anguilliformes (outgroup) falling into two clades. Within Perciformes, all the species under the same family were grouped together. The Moronidae species were placed as sister to the Lethrinidae. The Pomacanthidae and Chaetodontidae species were grouped as sister taxa. The four Morone and two Dicentrarchus species were clustered together with significant probability. Within Moronidae, the tree placed M. americana and M. mississippiensis as sister species and D. labrax sister to D. punctatus. The tree based on the concatenated nucleotide sequences of three mitochondrial genes showed similar results, with M. americana as sister taxon to M. mississippiensis, consistent with the conclusion of Leclerc et al. (Citation1999) based on repetitive nuclear DNA sequences. The mitogenome of M. americana represents a useful resource for marker development for population studies, as well as for studies of phylogeny of Moronidae and related species.
Disclosure statement
The authors declare no conflict of interest. The authors solely are responsible for the content and writing of the paper.
Funding information
This work was supported by Delaware Sea Grant [grant number NA14OAR4170087].
References
- Anderson S, Bankier AT, Barrell BG, De Bruijn M, Coulson AR, Drouin J, Eperon I, Nierlich D, Roe BA, Sanger F. 1981. Sequence and organization of the human mitochondrial genome. Nature. 290:457–465.
- Barnthouse LW, Glaser D, Desantis L. 2009. Polychlorinated biphenyls and Hudson River white perch: implications for population-level ecological risk assessment and risk management. Mol Phylogenet Evol. 5:435–444.
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogen E. 69:313–319.
- Cesaroni D, Venanzetti F, Allegrucci G, Sbordoni V. 1997. Mitochondrial DNA length variation and heteroplasmy in natural populations of the European sea bass, Dicentrarchus labrax. Mol Biol E. 14:560–568.
- Chapman RW. 1990. Mitochondrial DNA analysis of striped bass populations in Chesapeake Bay. Copeia. 2:355–366.
- Funderburk SL. 1991. Habitat requirements for Chesapeake Bay living resources. Solomons, Maryland (MD), USA: Chesapeake Research Consortium, Inc.
- Gruber AR, Lorenz R, Bernhart SH, Neuböck R, Hofacker IL. 2008. The Vienna RNA websuite. Syst Biol. 36:70–74.
- Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 59:307–321.
- Hiramatsu N, Matsubara T, Fujita T, Sullivan CV, Hara A. 2006. Multiple piscine vitellogenins: biomarkers of fish exposure to estrogenic endocrine disruptors in aquatic environments. Mol Biol Evol. 149:35–47.
- Iwasaki W, Fukunaga T, Isagozawa R, Yamada K, Maeda Y, Satoh TP, Sado T, Mabuchi K, Takeshima H, Miya M. 2013. MitoFish and MitoAnnotator: a mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Environ Sci Technol. 30:2531–2540.
- King RS, Beaman JR, Whigham DF, Hines AH, Baker ME, Weller DE. 2004. Watershed land use is strongly linked to PCBs in white perch in Chesapeake Bay subestuaries. Mar Biotechnol. 38:6546–6552.
- Leclerc GM, Han K, Leclerc GJ, Ely B. 1999. Characterization of a highly repetitive sequence conserved among the North American Morone species. Syst Biol. 1:122–130.
- Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61:539–542.
- Schattner P, Brooks AN, Lowe TM. 2005. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 5:686–689.
- Tine M, Kuhl H, Gagnaire PA, Louro B, Desmarais E, Martins RS, Hecht J, Knaust F, Belkhir K, Klages S, et al. 2014. European sea bass genome and its variation provide insights into adaptation to euryhalinity and speciation. Nat Commun. 5:5770.
- Williams E, Peer A, Miller T, Secor D, Place A. 2012 . A phylogeny of the temperate seabasses (Moronidae) characterized by a translocation of the mt-nd6 gene. J Fish Biol. 80:5770–130.
- Wirgin I, Pedersen M, Maceda S, Jessop B, Courtenay S, Waldman JR. 1995. Mixed-stock analysis of striped bass in two rivers of the Bay of Fundy as revealed by mitochondrial DMA. Can J Fish Aquat Sci. 52:961–970.
- Woolcott WS. 1962. Infraspecific variation in the white perch, Roccus americanus (Gmelin). Chesapeake Sci. 3:94–113.
- Wyman SK, Jansen RK, Boore JL. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 20:3252–3255.