Abstract
The classification of species originally assigned to the marine red algal genus Coeloseira G.J. Hollenberg remains univestigated using molecular data. Paired-end 36 bp Illumina sequences (Illumina Inc, San Diego, CA) were generated from a specimen of Coeloseira compressa G. J. Hollenberg and used to assemble the partial mitochondrial and complete plastid genomes. The mitogenome is 25,391 bp in length and contains 46 genes, and the plastome is 176,291 bp with 233 genes. Both organellar genomes show high gene synteny with previously published Florideophyceae. Comparison of organellar and nuclear phylogenetic markers (rbcL, CO1, LSU) of C. compressa to other genera in the Champiaceae supports its removal from Gastroclonium Kützing and the reinstatement of the name Coeloseira compressa.
The red algal order Rhodymeniales is a marine taxon distributed worldwide that consists of six families and about 50 genera (Schneider & Wynne Citation2007; Le Gall et al. Citation2008). Although phylogenetic studies of the Rhodymeniales based on gene sequences are published (Saunders et al. Citation1999; Le Gall et al. Citation2008), about 30% of the genera have yet to be analyzed. In addition, a plastid genome for the order has not been deciphered, and only one mitochondrial genome has been announced (Rhodymenia pseudopalmata (J.V. Lamouroux) P. C. Silva; GenBank KC875852; Kim et al. Citation2014). For this study, the marine red algal species Coeloseira compressa was analyzed. Hollenberg (Citation1941) originally assigned it to Coeloseira, but with a question mark because he lacked cystocarpic material. He noted, however, that the general habit and production of polyspores by C. compressa suggested a close relationship to the generitype, C. parva G. J. Hollenberg (Hollenberg Citation1941). According to Hollenberg, Coeloseira differed from Gastroclonium by the production of polyspores, rather than tetraspores. Smith (Citation1944) and later Abbott and Hollenberg (Citation1976) accepted Hollenberg’s placement of C. compressa in Coeloseira, but this opinion was rejected by Chang and Xia (Citation1978). The latter transferred C. compressa and C. parva to Gastroclonium, citing that some species of Gastroclonium, such as G. subarticulatum (Turner) Kützing, possess both polyspores and tetraspores, and that polyspores are derivatives of tetraspores. Hughey (Citation1995), Silva et al. (Citation1996), and Gabrielson et al. (Citation2004) accepted the name G. compressum (G.J. Hollenberg) Chang & Xia. Recent anatomical and molecular phylogenetic analyses on the Champiaceae (Le Gall et al. Citation2008) supported the transfer of G. subarticulatum to a new genus, Neogastroclonium. Neogastroclonium is distinguished from Gastroclonium by its solid erect axes and ostiolate cystocarps (Le Gall et al. Citation2008). Their study, however, did not analyze any species of Coeloseira.
To determine the mitochondrial and plastid genome structure of a member of the Champiaceae, and to address the taxonomic status of Coeloseira, a specimen of C. compressa was analyzed using next generation sequencing techniques. The DNA was extracted from a specimen from Tomales Bay, California (UC 2050599) following the protocol of Lindstrom et al. (Citation2011). The genomic library was constructed and sequenced by the High-Throughput Genomics Center (Seattle, Washington). The data were assembled using Velvet 1.2.08 (Zerbino & Birney Citation2008) on the Bio-Linux platform (Field et al. Citation2006) using the assembly and annotation methods described by Hughey et al. (Citation2014). Alignment of the Florideophyte mitogenomes was completed using default settings in MAFFT (Katoh & Standley Citation2013). The maximum likelihood analysis was performed using RaxML (Stamatakis Citation2014) with 1000 bootstrap replicates and default parameters in Galaxy (Giardine et al. Citation2005; Blankenberg et al. Citation2010; Goecks et al. Citation2010). The phylogenetic tree was generated with TreeDyn 198.3 at Phylogeny.fr (Dereeper et al. Citation2008).
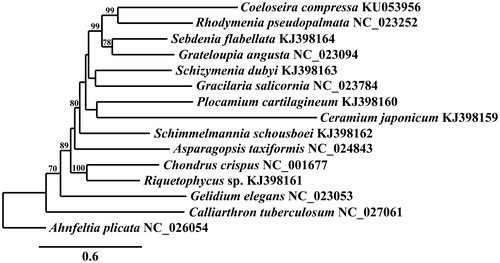
The partial mitogenome of C. compressa (GenBank KU053956) is 25,391 bp in length, AT rich (74.3%), and contains 46 genes including two ribosomal RNA genes (one rnl and one rns), 20 transfer RNAs, five ribosomal proteins (two rpl and three rps), ymf39 and 18 genes involved in electron transport and oxidative phosphorylation. The mitogenome of C. compressa is highly conserved compared to other Florideophyceae (Yang et al. Citation2015). It differs from R. pseudopalmata (GenBank accession no. KC875852) in the tRNAs present in the tandem tRNA region situated between atp6 and secY of the Florideophyte mitogenome. Coeloseira compressa contains trnSec, trnY, and a second copy of trnR, whereas R. pseudopalmata contains trnM and trnU, which are not present in C. compressa. Coeloseira compressa also lacks ORF-Rpse35. Phylogenetic analysis of the mitogenome of C. compressa resolves it in a fully supported clade with R. pseudopalmata in the Rhodymeniales, sister to Sebdenia flabellata (J. G. Agardh) P. G. Parkinson and Grateloupia angusta (Okamura) S. Kawaguchi & H. W. Wang ().
Figure 1. Maximum-likelihood phylogram of representative Florideophyceaen mitogenomes. Numbers along branches are RaxML bootstrap supports based on 1000 nreps (<70% support not shown). The legend represents the scale for nucleotide substitutions.
The complete plastid genome of C. compressa (GenBank KU053957) is 176,291 bp in length and contains 233 genes. The genome is AT rich (71.0%), and contains 19 small and 28 large ribosomal proteins, 29 photosystem I and II, 29 tRNA, 33 hypothetical chloroplast reading frames (ycf), 14 Open Reading Frames (ORFs), 10 phycobiliproteins, eight cytochrome b/f complex proteins, eight ATP synthase, three ribosomal RNAs and 62 other genes. Similar to all members of the Rhodymeniophycidae, C. compressa contains a single protochlorophyllide reductase gene (chlI). As found in Gracilaria tenustipitata var. liui Zhang & Xia (GenBank NC_006137; Hagopian et al. Citation2004), C. compressa codes for ycf23 and ycf86, but lacks ycf57.
Phylogenetic analysis of C. compressa with other Champiaceae using standard genetic markers (rbcL, CO1, and LSU GenBank accession no. KU052799) indicates that C. compressa is polyphyletic with respect to the generitype of Gastroclonium, G. ovatum (Hudson) Papenfuss. Calculation of intergeneric pairwise sequence divergences between representative Champiaceae found that C. compressa is most closely related to N. subarticulatum. Coeloseira compressa differs from N. subarticulatum by 5.7% (rbcL), 11.0% (CO1) and 1.5% (LSU). These percentages are within interspecific ranges typically reported for red algae, suggesting that Coeloseira and Neogastroclonium are congeneric. Analysis of the generitype of Coeloseira is required to determine the fate of the more recent name Neogastroclonium.
Disclosure statement
The authors wish to thank Dr. Carla Fresquez at the Undergraduate Research Opportunities Center (UROC) at California State University, Monterey Bay, as well as UROC and a private family trust from Paul W. Gabrielson for financial support.
References
- Abbott IA, Hollenberg GJ. 1976. Marine algae of California. Stanford (CA): Stanford University Press.
- Blankenberg D, Von Kuster G, Coraor N, Ananda G, Lazarus R, Mangan M, Nekrutenko A, Taylor J. 2010. Galaxy: a web-based genome analysis tool for experimentalists. Curr Protoc Mol Biol Ch. 19, Unit 19.10:11–21.
- Chang CF, Xia BM. 1978. A new species of Gastroclonium from the Xisha Islands, Guangdong Province, China. Oceanol Limnol Sin. 9:209–214.
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, et al. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36:W465–W469.
- Field D, Tiwari B, Booth T, Houten S, Swan D, Bertrand N, Thurston M. 2006. Open software for biologists: from famine to feast. Nat Biotechnol. 24:801–803.
- Gabrielson PW, Widdowson TB, Lindstrom SC. 2004. Keys to the seaweeds and seagrasses of Oregon and California: North of Point Conception. Phycological Contribution, vol. 6. Hillsborough (NC): Paul W Gabrielson.
- Giardine B, Riemer C, Hardison RC, Burhans R, Elnitski L, Shah P, Zhang Y, Blankenberg D, Albert I, Taylor J, et al. 2005. Galaxy: a platform for interactive large-scale genome analysis. Genome Res. 15:1451–1455.
- Goecks J, Nekrutenko A, Taylor J, The Galaxy Team. 2010. Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. 11:R86.
- Hagopian J, Reis M, Kitakima J, Bhattacharya D, de Oliveira MC. 2004. Comparative analysis of the complete chloroplast genome of the red alga Gracilaria tenuistipitata var. liui provides insights into the evolution of rhodoplasts and their relationship to other chloroplasts. J Mol E. 59:464–477.
- Hollenberg GJ. 1941. New marine algae from southern California. I. Am J Bot. 27:868–877.
- Hughey JR. 1995. Noteworthy collections. California. Madroño 42:409.
- Hughey JR, Gabrielson PW, Rohmer L, Tortolani J, Silva M, Miller KA, Young JD, Martell C, Ruediger E. 2014. Minimally destructive sampling of type specimens of Pyropia (Bangiales, Rhodophyta) recovers complete plastid and mitochondrial genomes. Sci Rep. 4:5113.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780.
- Kim KM, Yang EC, Yi G, Yoon HS. 2014. Complete mitochondrial genome of sublittoral macroalga Rhodymenia pseudopalmata (Rhodymeniales, Rhodophyta). Mitochondr DNA. 25:273–274.
- Le Gall L, Dalen JL, Saunders GW. 2008. Phylogenetic analyses of the red algal order Rhodymeniales supports recognition of the Hymenocladiaceae fam. nov., Fryeellaceae fam. nov., and Neogastroclonium gen. NOV.(1). J Phycol. 44:1556–1571.
- Lindstrom SC, Hughey JR, Martone PT. 2011. New, resurrected and redefined species of Mastocarpus (Phyllophoraceae, Rhodophyta) from the northeast Pacific. Phycologia. 50:661–683.
- Saunders GW, Strachan IM, Kraft GT. 1999. The families of the order Rhodymeniales (Rhodophyta): a molecular-systematic investigation with a description of Faucheaceae fam. nov. Phycologia. 38:23–40.
- Schneider CW, Wynne MJ. 2007. A synoptic review of the classification of red algal genera a half a century after Kylin’s ‘‘Die Gattungen der Rhodophyceen’’. Bot Mar. 50:197–249.
- Silva PC, Basson PW, Moe RL. 1996. Catalogue of the benthic marine algae of the Indian Ocean. Univ Calif Publ Bot. 79:1–1259.
- Smith GM. 1944. Marine algae of the Monterey Peninsula. Stanford (CA): Stanford University Press.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30:1312–1313.
- Yang EC, Kim KM, Kim SY, Lee J, Boo GH, Lee JH, Nelson WA, Yi G, Schmidt WE, Fredericq S, et al. 2015. Highly conserved mitochondrial genomes among multicellular red algae of the florideophyceae. Genome Biol Evol. 7:2394–2406.
- Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829.
