Abstract
The chloroplast genome of the tree fern Alsophila podophylla has been completely sequenced. The genome is 166,151 bp in size and features a typical quadripartite structure with the large (LSC, 86,762 bp) and small single copy (SSC, 21,641 bp) regions separated by a pair of inverted repeats (IRs, 28,874 bp each). It encodes 133 genes including 91 protein-coding genes, 33 tRNA genes, eight rRNA genes, and one pseudogene. Maximum-likelihood tree indicates that A. podophylla is sister to A. spinulosa. This work provides a solid molecular resource for surveying phylogeny and chloroplast genomics of ferns.
Alsophila podophylla Hooker is a tree fern belonging to subgenus Gymnosphaera (genus Alsophila, Cyatheaceae), with a trunk height of 1–3 m (Zhang and Nishida Citation2013). It can distinguish from other tree ferns by its nearly entire pinnules, basal pinna with a long stalk, and lamina with a conform terminal pinna. As a relic species, the distribution range of A. podophylla had drastically reduced during the Quaternary glaciation events (Zhou et al. Citation2004; Su et al. Citation2005). In China, its extant individuals are mainly restricted to rain forests at an altitude of 350–700 m (Su et al. Citation2005; Zhang and Nishida Citation2013). Alsophila podophylla represents a core member to deal with the classification and phylogeny of Cyatheaceae (Ching Citation1978; Wang et al. Citation2003), acquirement of its whole chloroplast (cp) genome sequence will facilitate the chloroplast phylogenomics of Cyatheaceae.
Fresh leaves of A. podophylla were sampled from the living collection at South China Botanical Garden, Chinese Academy of Sciences (CAS). The specimen is stored in Herbarium of Sun Yat-sen University (SYS; voucher: SS Liu 201610). Genomic DNA was extracted using the Tiangen Plant Genomic DNA Kit (Tiangen Biotech Co., Beijing, China). Sequencing took place on the Hiseq 2500 platform (Illumina Inc., San Diego, CA), which generated a total of 7,222,454 raw reads. After quality assessment, clean reads were de novo assembled into contigs by Velvet (Zerbino and Birney Citation2008), which were further aligned and oriented with the cp genome of Woodwardia unigemmata (NC_028543) as a reference. All gaps were filled by PCR amplification. Annotations were performed using DOGMA (Wyman et al. Citation2004) and tRNAscan-SE programs (Lowe and Eddy Citation1997). The program MAFFT v7.311 (Katoh and Standley Citation2013) was used to create a multiple sequence alignment of the complete cp genome of A. podophylla with those of other nine plants downloaded from GenBank. A phylogenetic tree based on maximum-likelihood (ML) analysis was inferred using RAxML v.8.0 with 1000 bootstrap replicates (Stamatakis Citation2014).
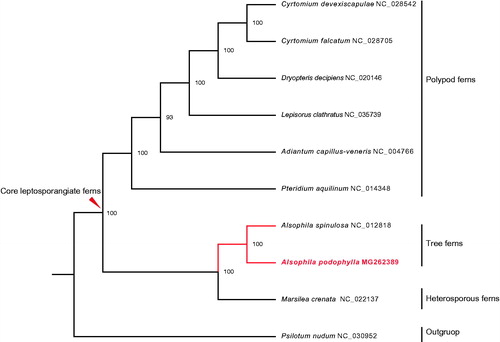
The complete cp genome of A. podophylla is a circular DNA molecule of 166,151 bp in length (GenBank accession number: MG262389), which is the largest plastome among the studied ferns so far. It has a typical quadripartite structure with the large (LSC, 86,762 bp) and small single copy (SSC, 21,641 bp) regions separated by two identical inverted repeats (IRs, 28,874 bp each). The cpDNA contains 133 genes, including 91 protein-coding genes, eight ribosomal RNA genes, 33 tRNA genes, and one pseudogene. Among these genes, 118 are unique genes, and 14 are totally duplicated in IRs. The ndhB gene in IRb was identified as a pseudogene due to a fragment duplication in exon 2. In addition, 14 genes contain one intron, whereas three genes (ycf3, clpP, and rps12) have two introns. ML tree strongly supports that A. podophylla is the sister group to A. spinulosa (); and they further form a monophyletic clade with the heterosporous fern Marsilea crenata. The cp genome of A. podophylla provides a reliable molecular resource for phylogenetic studies and chloroplast genomics of ferns.
Disclosure statement
The authors declare no conflict of interests. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Ching RC. 1978. The Chinese fern families and genera: systematic arrangement and historical origin. Acta Phytotaxon Sin. 16:1–19. (in Chinses).
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780
- Lowe TM, Eddy SR. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25:955–964.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30:1312–1313.
- Su YJ, Wang T, Zheng B, Jiang Y, Ouyang PY, Chen GP. 2005. Genetic variation and phylogeographical patterns in Alsophila podophylla from southern China based on cpDNA atpB-rbcL sequence data. Amer Fern Jour. 95:68–79.
- Wang T, Su YJ, Zheng B, Li XY, Chen GP, Zeng QL. 2003. Phylogenetic analysis of the chloroplast trnL intron and trnL-trnF intergenic spacer sequences of the Cyatheaceae plants from China. J Trop Subtrop Bot. 11:137–142. (in Chinese).
- Wyman SK, Jansen RK, Boore JL. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 20:3252–3255.
- Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829.
- Zhang XC, Nishida H. 2013. Cyatheaceae. In: Wu ZY, Raven PH, Hong DY, editors. Flora of China. Vol.2–3 (Pteridophytes). Beijing: Science Press; p. 134–138.
- Zhou ZQ, Su ZX, Liao YM, Su RJ, Li YX. 2004. Advance of biological study on Alsophila spinulosa. J Guizhou Normal Univ (Nat Sci). 22:100–103. (in Chinese).