Abstract
The Allegheny woodrat (Neotoma magister) is endemic to the eastern United States. Population numbers have decreased rapidly over the last four decades due to habitat fragmentation, disease-related mortality, genetic isolation and inbreeding depression; however, effective management is hampered by limited genetic resources. To begin addressing this need, we sequenced and assembled the entire Allegheny woodrat mitochondrial genome. The genome assembly is 16,310 base pairs in length, with an overall base composition of 34% adenine, 27% thymine, 26% cytosine and 13% guanine. This resource will facilitate our understanding of woodrat population genetics and behavioral ecology.
Keywords:
The Allegheny woodrat (Neotoma magister) is endemic to eastern North America, where population numbers have decreased rapidly since the 1980s (Wright Citation2008). Causes of the decline include habitat destruction, decreased food availability, increased mortality caused by Baylisascaris procyonis infection, genetic isolation and inbreeding depression (LoGiudice Citation2008; Smyser et al. Citation2012). Despite this threatened status, genetic studies have been limited (Castleberry et al. Citation2000, Citation2002; Smyser et al. Citation2012) and NCBI cites just 59 archived N. magister nucleotide sequences. In contrast, 369 and 1567 nucleotide sequences have been archived for the dusky-footed woodrat (N. fuscipes) and the Mexican woodrat (N. mexicana), respectively. Assembling the complete N. magister mitogenome will provide important resources for species identification (e.g., COI sequence) and contribute to our understanding of population structure across the species range.
We assembled the mitogenome of a single N. magister individual sampled in Westmoreland County, Pennsylvania (latitude/longitude (40.3, −79.3)). The sample is stored at −80 °C at Towson University in Baltimore County, Maryland (accession number J5). DNA was extracted with commercially available extraction (DNEasy Blood and Tissue, Qiagen, Venlo, the Netherlands) and clean-up (DNA Clean & Concentrator, Zymo Research, Irvine, California) kits, according to the instructions from the manufacturers. We conducted one lane of paired-end sequencing using an Illumina HiSeq2000 and used Trimmomatic (Bolger et al. Citation2014) to remove adaptors, discard short reads (<30 bp), and trim poor quality bases (Illumina Q-value ≤20) from both 5′ and 3′ ends of raw sequence reads.
We used a 12S rRNA sequence (accession number DQ179706.1) to seed mitogenome assembly implemented by MITObim and MIRA (baiting-and-iterative mapping; Chevreux et al. Citation1999; Hahn et al. Citation2013). The draft assembly was annotated using MITOS (Bernt et al. Citation2013). We used CLUSTALW implemented by MEGA 7.0.21 (Tamura et al. Citation2007) to align mitogenomes from N. magister, 19 other Cricetid species and an outgroup (Sciurus vulgaris) . This alignment was used to produce a maximum-likelihood tree using the GTR + G + I model of evolution and 1000 bootstraps (Figure 1).
Figure 1. We used CLUSTALW implemented by MEGA 7.0.21 to align the mtDNA genome sequences from N. magister, N. fuscipes, N. mexicana (the only other woodrats for which complete mitogenome sequences were available from NCBI), 17 other Cricetid species and an outgroup (Sciurus vulgaris). This alignment was used to produce a maximum likelihood tree using the GTR + G + I model of evolution and 1000 bootstraps. Bootstrap values are included at each node and each species label includes the GenBank accession number, with the exception of N. magister (GenBank accession number MG182016).
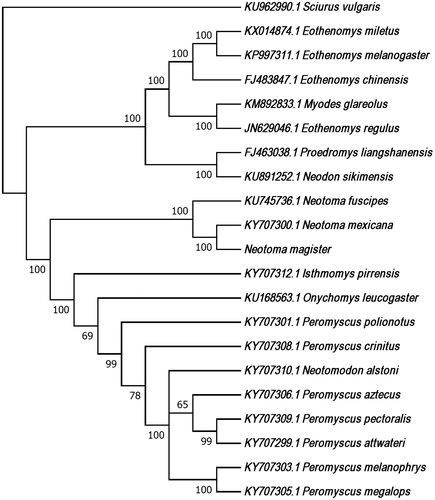
The N. magister mitogenome (GenBank accession number MG182016) is 16,310 bp in length with 22 tRNAs, 2 rRNA genes and 13 protein-coding genes. Overall base composition includes 34% adenine, 27% thymine, 26% cytosine and 13% guanine. Gene order and organization are identical to that of N. fuscipes (Brown and Blois Citation2016) and N. mexicana (Sullivan et al. Citation2017). Our phylogeny indicates that N. magister is more closely related to N. mexicana than to N. fuscipes, a result consistent with a phylogeny generated using a combination of mitochondrial and nuclear markers (Matocq et al. Citation2007). Furthermore, as in Reeder et al. (Citation2006), the clade including Neotoma species is sister to the clade including Neotomodon, Onychomys, and Peromyscus (i.e., Peromyscini).
This new genetic resource has important management implications. For example, our annotation of conserved mitochondrial genes will aid species identification from noninvasively collected samples. In addition, this mitogenome assembly may facilitate haplotyping across the N. magister range (e.g., by improving primer design), expanding upon our understanding of population structure and allowing us to identify evolutionarily significant units.
Disclosure statement
We have no conflicts of interest to report and the authors are solely responsible for the content and writing of this paper.
Additional information
Funding
References
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69:313–319.
- Bolger A, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30:2114–2120.
- Brown SK, Blois JL. 2016. The complete mitochondrial genome of the dusky-footed woodrat (Neotoma fuscipes) (Rodentia, Cricetidae). Mitochondrial DNA. 1:728–729.
- Castleberry SB, King TL, Wood PB, Ford WM. 2000. Microsatellite DNA markers for the study of Allegheny woodrat (Neotoma magister) populations and cross-species amplification in the genus Neotoma. Mol Ecol. 9:824–826.
- Castleberry SB, King TL, Wood PB, Ford WM. 2002. DNA analysis of population structure in Allegheny woodrats (Neotoma magister). J Mammal. 83:1058–1070.
- Chevreux B, Wetter T, Suhai S. 1999. Genome sequence assembly using trace signals and additional sequence information. Computer Science and Biology: Proceedings of the German Conference on Bioinformatics, Vol. 99:45–56.
- Hahn C, Bachmann L, Chevreux B. 2013. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads – a baiting and iterative mapping approach. Nucleic Acids Res. 41:e129–e129.
- LoGiudice K. 2008. Multiple causes of the Allegheny woodrat decline: a historical–ecological examination. New York (NY): Springer.
- Matocq MD, Shurtliff QR, Feldman CR. 2007. Phylogenetics of the woodrat genus Neotoma (Rodentia: Muridae): Implications for the evolution of phenotypic variation in male external genitalia. Mol Phylogenet Evol. 42:637–652.
- Smyser TJ, Johnson SA, Page LK, Rhodes OE Jr. 2012. Synergistic stressors and the dilemma of conservation in a multivariate world: a case study in Allegheny woodrats. Animal Conservation. 15:205–213.
- Sullivan KAM, Platt RN, Bradley RD, Ray DA. 2017. Whole mitochondrial genomes provide increased resolution and indicate paraphyly in deer mice. BMC Zool. 2:11.
- Reeder SA, Carroll DS, Edwards CW, Kilpatrick CW, Bradley RD. 2006. Neotomine–peromyscine rodent systematics based on combined analyses of nuclear and mitochondrial DNA sequences. Mol Phylogenet Evol. 40:251–258.
- Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 24:1596–1599.
- Wright J. 2008. History and current status of the Allegheny woodrat. In: Peles JD, Wright J, editors. The Allegheny woodat: ecology, conservation and management of a declining species. New York (NY): Springer; p. 29–40.
