Abstract
The complete mitochondrial genome sequence of cone snail Conus quercinus a kind of worm-hunting sea snails, was performed by next-generation sequencing. The mitogenome is 16,439 bp in length, including 13 protein-coding genes, 22 tRNA genes, two ribosomal RNA genes (12S and 16S rRNA), and one control region. It has overall base composition of A (28.1%), T (38.2%), C (14.7%), and G (18.6%). It shows 75.9% identity with C. capitaneus, which also belongs to worm-hunting sea snail. The phylogenetic analysis was conducted with 22 closely related species to assess their phylogenetic relationship. The complete mitogenome of the C. quercinus provides important DNA molecular data for further phylogeography.
Cone snails are common names for a large group of venomous predatory sea snails and marine gastropod molluscs. There are over 600 species of cone snails classified under four kinds of genus, including Conus, Conasprella, Profundiconus, and Californiconus (Puillandre et al. Citation2015). All of them are in one family, the Conidae. Based on their prey preference, cone snails can be divided into three groups, piscivorous, molluscivorous, and vermivorous (Olivera Citation1997; Le Gall et al. Citation1999). There are approximately 30 records of humans killed by cone snails. Human victims suffer little pain, because the venom contains an analgesic component (Nelson Citation2004).
Conus quercinus (Lightfoot Citation1786), a kind of vermivorous (worm-hunting) sea snail, also names the oak cone (Carpenter and Niem Citation1998). Their sizes are between 60 mm and 140 mm (Röckel et al. Citation1995). This species usually distribute in the Indo-West Pacific, from East Africa to eastern Polynesia; north to Japan and Hawaii, and south to Queensland and New Caledonia. They usually bury in the sand during the day but actively foraging for food during evening (Carpenter and Niem Citation1998).
The specimens of C. quercinus (voucher no. 20150421-029; with GenBank accession no. MH400188) in this study were collected from Penghu, Taiwan (23.565N, 119.576E). The samples were deposited in Marine Toxins Lab., Department of Food Science, National Taiwan Ocean University, Taiwan. The total genomic DNA was extracted from muscle using magnetic bead technique with the KingFisher magnetic processors (ThermoFisher Scientific Inc., Worcester, MA). The raw next-generation sequencing reads generated from MiSeq sequencer (Illumina, San Diego, CA) were de novo assembled and reference mapping was conducted by commercial software (Geneious V11, Auckland, New Zealand) to produce a single circular form of complete mitogenome with about an average 45.2 coverage (3114 out of 11,798,800 reads, 0.026%). The complete mitochondrial genome of C. quercinus is 16,439 bp in size, including 13 protein-coding genes, 22 tRNA genes, two ribosomal RNA genes (12S and 16S rRNA), and one control region. The overall base composition of C. quercinus is 28.1% for A, 38.2% for T, 14.7% for C, and 18.6% for G. It shows 75.9% identity with C. capitaneus (KX155573), which is also a worm-hunting cone snail. The protein coding rRNA and tRNA genes of C. quercinus mitogenome were predicted by using MITOS (Bernt et al. Citation2013) and tRNAscan-SE (Schattner et al. Citation2005).
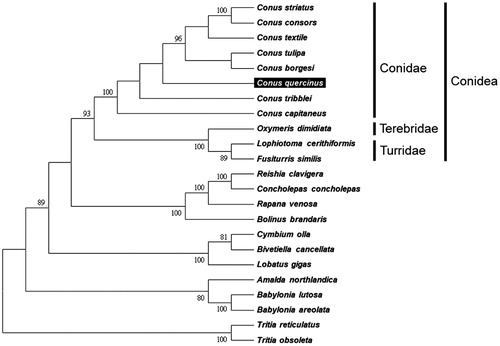
We used MEGA 6 (Tamura et al. Citation2013) to construct the phylogenetic relationships of the C. quercinus and related families by neighbour-joining method with 1000 bootstrap replicates based on the 13 protein-coding genes and two ribosomal RNA genes of the other 22 complete mitochondrial genomes of Neogastropoda sea snails, which are reported in GenBank of NCBI database. Bootstrap support values were relatively high, with 10 nodes having values >95%, and nine nodes demonstrating 100% bootstrap support (). C. quercinus was grouped together with seven other cone snails from the family Conidae. The lineages of Conidae strongly supported in this report and agreed with previous studies (Bouchet et al. Citation2011; Puillandre et al. Citation2014).
Figure 1. Phylogenetic tree generated using the neighbour-joining method based on complete mitochondrial genomes. Conus striatus (KX156937), C. consors (KF887950), C. textile (DQ862058), C. tulipa (KR006970), C. borgesi (EU827198), C. quercinus (MH400188), C. tribblei (KT199301), C. capitaneus (KX155573), Oxymeris dimidiata (EU827196), Lophiotoma cerithiformis (DQ284754), Fusiturris similis (EU827197), Reishia clavigera (DQ159954), Concholepas concholepas (JQ446041), Rapana venosa (KM213962), Bolinus brandaris (EU827194), Cymbium olla (EU827199), Bivetiella cancellata (EU827195), Lobatus gigas (KM245630), Amalda northlandica (GU196685), Babylonia lutosa (KF897830), B. areolata (HQ416443), Tritia reticulatas (EU827201) and Tritia obsoleta (DQ238598).
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69:313–319.
- Bouchet P, Kantor Y, Sysoev A, Puillandre N. 2011. A new operational classification of the Conidea (Gastropoda). J Molluscan Stud. 77:273–308.
- Carpenter KE, Niem VH, editors. 1998. FAO species identification guide for fishery purposes. The living marine resources of the Western Central Pacific. Volume 1: Seaweeds, corals, bivalves and gastropods. Rome: Food and Agriculture Organization of the United Nations; p. 618–628.
- Le Gall F, Favreau P, Richard G, Letourneux Y, Molgo J. 1999. The strategy used by some piscivorous cone snails to capture their prey: the effects of their venoms on vertebrates and on isolated neuromuscular preparations. Toxicon. 37:985–998.
- Lightfoot J. 1786. A catalogue of the Portland Museum, lately the property of the Duchess Dowager of Portland: deceased which will be sold by auction. London: Mr. Skinner and Co.; viii, 194 pp.
- Nelson L. 2004. Venomous snails: one slip, and you're dead. Nature. 429:798–799.
- Olivera BM. 1997. E.E. Just Lecture, 1996. Conus venom peptides, receptor and ion channel targets, and drug design: 50 million years of neuropharmacology. Mol Biol Cell. 8:2101–2109.
- Puillandre N, Bouchet P, Duda TF, Kauferstein S, Kohn AJ, Olivera BM, Watkins M, Meyer C. 2014. Molecular phylogeny and evolution of the cone snails (Gastropoda, Conoidea). Mol Phylogenet Evol. 78:290–303.
- Puillandre N, Duda TF, Meyer C, Olivera BM, Bouchet P. 2015. One, four or 100 genera? A new classification of the cone snails. J Molluscan Stud. 81:1–23.
- Röckel D, Korn W, Kohn AJ. 1995. Manual of the Living Conidae. Volume 1: Indo-Pacific Region. Germany: Verlag Christa Hemmen; p. 133–135.
- Schattner P, Brooks AN, Lowe TM. 2005. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 33:W686–W689.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics Analysis version 6.0. Mol Biol Evol. 30:2725–2729.
